ABSTRACT
Denosumab is one of the most commonly used antiresorptive drugs for osteoporosis treatment and the prevention of skeletal-related events in cancer patients. The purpose of this case report is to highlight potentially life-threatening severe hypocalcaemia as a side effect of denosumab complicated by refractory shock that failed to respond to medical management including intravenous calcium, vasopressors and inotropes in an elderly man with a history of prostatic cancer.
LEARNING POINTS
- Denosumab is a commonly used antiresorptive drugs for the treatment of osteoporosis and to prevent skeletal-related events in patients with cancer.
- A common side effect of denosumab is hypocalcaemia; conditions associated with a higher risk of hypocalcaemia include chronic kidney disease, pre-existing hypocalcaemia, and metastatic cancer.
- Severe hypocalcaemia may induce cardiovascular manifestations such as hypotension, bradycardia, impaired cardiac contractility, impaired vascular tone, and arrhythmias.
- Shock results from diminished vascular smooth muscle tone and tends to occur with rapid severe hypocalcaemia; it is usually refractory to fluid and pressor therapy until hypocalcaemia is corrected.
KEYWORDS
Refractory shock, cardiogenic shock, denosumab, severe hypocalcaemia
INTRODUCTION
Denosumab is a human monoclonal antibody against receptor activator of nuclear factor κB ligand (RANKL), which acts by inhibiting the formation and function of osteoclasts. This results in the regulation of bone turnover with decreased bone resorption and increases in bone mass and strength. It is most commonly used for the treatment of osteoporosis; other indications include treatment of bone loss in high-risk patients such as those receiving androgen deprivation therapy or aromatase inhibitors, hypercalcaemia of malignancy, and prevention of skeletal-related events in metastatic cancer [1]. Hypocalcaemia has been commonly reported with denosumab use; risk factors include renal impairment, vitamin D deficiency, and malignancy. Herein, the authors describe a patient with refractory shock secondary to denosumab-induced severe hypocalcaemia.
CASE DESCRIPTION
A 78-year-old man presented to the emergency department complaining of a 4-day history of severe weakness, anorexia, nausea and vomiting. He denied having fever, cough, diarrhoea, constipation or abdominal pain. His past medical history was significant for hypertension, chronic kidney disease (CKD) stage 4, and prostate cancer which was managed with surgery, radiotherapy and androgen deprivation therapy. His medications included amlodipine and enzalutamide and he had received denosumab 2 weeks prior to this presentation for osteoporosis prophylaxis.
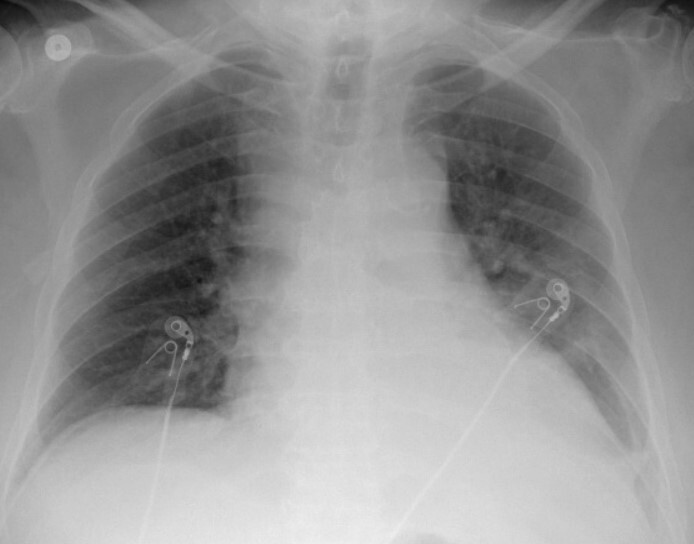
On initial clinical examination, he was afebrile with a temperature of 36.7°C, hypotensive with a blood pressure of 85/55 mmHg, tachycardiac with a heart rate of 90 beats per minute, and a respiratory rate of 18 per minute. He had dry mucous membranes, his chest was clear to auscultation, and his heart sounds were normal. Initial laboratory results revealed leucocytosis with neutrophilia with a white blood cell (WBC) count of 18×103 ml, acute kidney injury on top of CKD stage 4 with a creatinine of 3.5 mg/dl from a baseline of 2.7 mg/dl, high anion gap metabolic acidosis with positive acetone in the blood, and profound hypocalcaemia with a corrected calcium level of 3.9 mg/dl and ionized calcium of 0.65 mmol/ml. His initial troponin and pro-B-type natriuretic peptide (BNP) were mildly elevated and procalcitonin was negative, urine analysis was unremarkable apart from +1 proteins, and chest x-ray showed cardiomegaly but no pulmonary infiltrates (Fig. 1). An electrocardiogram (EKG) showed normal sinus rhythm with a heart rate of 90 beats per minute and no other EKG changes. His previous echocardiogram a few months previously showed a normal left ventricular ejection fraction (LVEF) of 70%. The initial differential diagnosis included hypovolaemic shock based on his history of anorexia and vomiting with starvation ketosis versus septic shock.
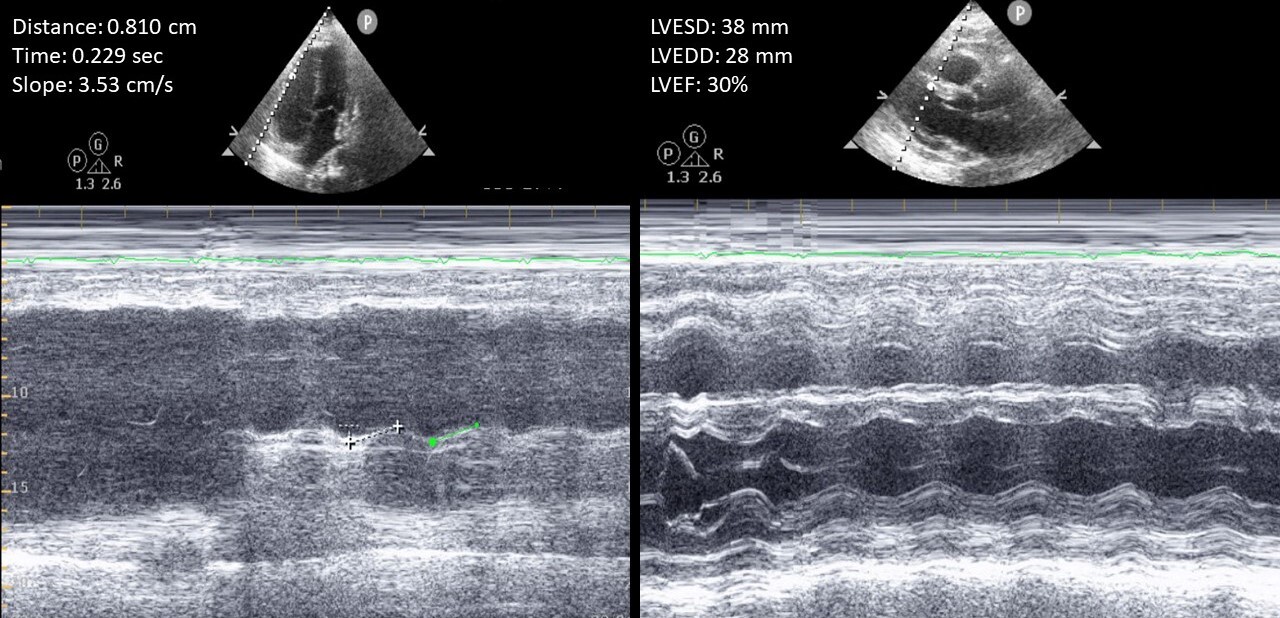
Resuscitation measures were carried out with intravenous (IV) fluids, calcium gluconate infusion, and empirical broad-spectrum antibiotics, and the patient was admitted to the intensive care unit. With calcium infusion, his calcium level slowly improved, and corrected calcium increased to 4.8 and then 5.7 mg/dl. Ionized calcium however remained low at 0.65 mmol/ml. The rest of his laboratory work-up showed hyperphosphataemia of 8.2 mg/dl, hyperparathyroidism with a PTH of 399 pg/ml, and severe vitamin D deficiency of 9 ng/ml (>30). Two weeks previously, at the time of denosumab injection, his laboratory results showed calcium of 9.2 mg/dl, phosphorus of 4.5 mg/dl, and PTH of 65 pg/ml. He continued to be hypotensive and acidotic, with an initially normal lactic acid level trending up. His blood cultures showed no growth. For proper haemodynamic assessment, central and arterial lines and a Foley catheter were placed with minimal urine output. He was started on norepinephrine, followed by vasopressin and phenylephrine for haemodynamic support. An urgent transthoracic echocardiography (TTE) was performed showing new onset biventricular systolic dysfunction with global hypokinesia with an LVEF of 30% and tricuspid annular plane systolic excursion (TAPSE) of 0.8 cm (Fig. 2).
Inotropic support in the form of dobutamine infusion was started, and the diagnosis was revised to hypocalcaemia-induced cardiogenic shock and vasoplegia without any significant improvement. Due to his deteriorated condition, no further invasive diagnostic or therapeutic procedures for further haemodynamic monitoring were carried out, or mechanical circulatory support provided per his family’s request. Despite all haemodynamic supportive measures, he died on the second day of hospitalization.
Figure 2.Transthoracic echocardiography showing biventricular systolic dysfunction with a left ventricular ejection fraction of 30% and tricuspid annular plane systolic excursion of 0.8 cm
DISCUSSION
Denosumab is a fully human monoclonal antibody against RANKL which prevents it from binding to RANK receptors on the osteoclast surface, decreasing bone resorption and increasing bone mass [1]. Hypocalcaemia is a common side-effect of denosumab, reported in around 2% of patients in clinical trials but up to 40% in the literature post-marketing [2, 3]. It usually develops 7–14 days following administration. Certain conditions are associated with a higher risk of developing hypocalcaemia, including CKD, with a lower glomerular filtration rate (GFR) associated with an increased likelihood of hypocalcaemia, pre-existing hypocalcaemia, and metastatic cancer [3].
This patient had several risk factors for the development of hypocalcaemia, including CKD stage 4, vitamin D deficiency and prostatic cancer, and was not properly supplemented with calcium and vitamin D. His work-up did not reveal alternative causes of shock, there was no apparent source of infection, and the patient had a negative routine cardiac work-up 2 weeks prior to hospitalization. Coronary angiography was proposed to rule out the presence of concomitant coronary artery disease, but his family decided not to proceed with any invasive procedures. There was a temporal relationship between hypocalcaemia and the abrupt onset of his symptoms.
Severe hypocalcaemia may induce cardiovascular manifestations such as hypotension, bradycardia, impaired cardiac contractility, and arrhythmias. Hypotension results from diminished vascular smooth muscle tone and tends to occur when the rate of fall of serum calcium is extremely rapid. It is generally refractory to fluid and pressor therapy until hypocalcaemia is corrected [4]. Olson et al. reported severe hypocalcaemia as a cause of cardiogenic shock in a patient with pheochromocytoma, where interruption of calcium infusion resulted in hypotension and tachycardia [5]. Other cases were reported of severe prolonged life-threatening hypocalcaemia in patients with metastatic prostate cancer [6].
The authors propose that refractory shock in this patient was the result of severe hypocalcaemia, not corrected by calcium infusion (ionized calcium remained very low) which blunted inotropic and vasopressor response. In patients with risk factors, the risks versus benefits of denosumab administration should be discussed with the patient. Ensuring normal vitamin D and calcium levels before initiation of therapy, as well as continuous supplementation of calcium and vitamin D during therapy, are important steps to try to prevent or ameliorate ensuing hypocalcaemia.
CONCLUSION
Clinicians should be aware that severe hypocalcaemia can cause cardiogenic shock refractory to fluid and pressor resuscitation until hypocalcaemia is corrected. The risks versus benefits of denosumab administration should be discussed with patients with risk factors for hypocalcaemia.