ABSTRACT
Clostridioides (formerly Clostridium) difficile infection is a common and costly healthcare-associated infection. Extraintestinal C. difficile infection is rarely encountered, especially in isolation. We present a unique case of abdominal wall abscess presenting six months following gastrointestinal (GI) surgery. The patient was managed with computed tomography (CT) guided drainage of the abscess, placement of a drainage catheter, and aggressive broad-spectrum antibiotic treatment for a prolonged duration over multiple admissions.
LEARNING POINTS
- Risk factors for extraintestinal CDI include prior hospital stay, prolonged antibiotic therapy, proton pump inhibitor (PPI) use, relative state of immunodeficiency such as malnutrition and diabetes mellitus, previous abdominal surgery especially following perforation and leak of intestinal content.
- Presentation can be late following surgery with mesh repair (foreign body implantation) for intestinal perforation as they have high risk of colonisation, which later leads to infection.
- For extraintestinal CDI in the presence of a foreign body, removal is the desired course of action. But it is not always possible given the presence of comorbidities in this population, thus resulting in a prolonged course of antibiotics.
KEYWORDS
Extraintestinal CDI, abdominal wall abscess, persistent C. difficile abscess
INTRODUCTION
Clostridioides difficile infection (CDI) is the most prevalent healthcare-related infection, increasing morbidity and mortality in hospitalised patients. Up to 29,000 deaths from community-acquired sources have been reported in the literature[1]. Likewise, as this nosocomial infection continues to increase, healthcare expenditures of up to $1.5 billion have been estimated in the United States[1]. In contrast to intestinal CDI, extraintestinal CDI is a rarer occurrence[2]. Extraintestinal CDI has been described in the small intestine as cellulitis, as a proponent in reactive arthritis, visceral abscess formation, septic arthritis, and empyema[3-5]. Among extraintestinal CDI, postoperative intra-abdominal abscess was commonly reported[2]. Our patient had persistent abdominal wall abscess with remote partial ileal resection and subsequent monomicrobial C. difficile infection; an extremely uncommon occurrence. Furthermore, there are no well-defined guidelines for treatment of extraintestinal CDI, unlike the Infectious Disease Society of America (IDSA) guidelines for intestinal CDI[6]. Thus, treatment remains a challenge. Here, we present an eccentric case of extraintestinal CDI of the abdominal wall and the difficulties faced in treating the patient.
CASE DESCRIPTION
A 61-year-old African American female, who was a resident of a long-term care facility with a history of multiple comorbidities, presented to our institution with altered mental status. Her medical history included type 2 diabetes mellitus, essential hypertension, gastroesophageal reflux disease on chronic proton pump inhibitors, and end-stage renal disease (ESRD) on haemodialysis. Medical history was negative for intestinal CDI, malignancies, and liver disease. Pertinent surgical history included perforated diverticulitis status post colectomy with colostomy 20 years ago, a history of three ventral hernias and a strangulated parastomal hernia with small bowel perforation six months ago requiring partial ileal resection. There were multiple intra-abdominal washouts, and an AbThera™ open abdomen dressing (3M™, St Paul, MN) for seven days before complete abdominal and ostomy closure with mesh, for which she was also treated with multiple antibiotics. Significant current medications include lansoprazole.
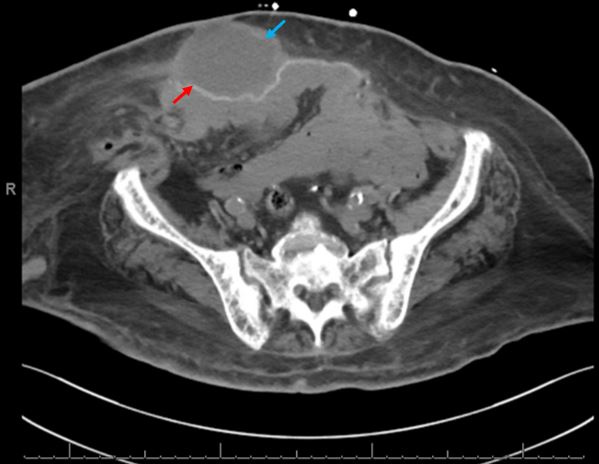
In the emergency department the patient was encephalopathic, oriented to self and place. She was found to be in shock requiring fluid resuscitation and vasopressor support. Abdominal examination revealed abdominal wall induration and erythema around the midline surgical scar spanning 4–5 cm bilaterally with overlying skin crusting and desquamation. Follow-up computed tomography (CT) of the abdomen and pelvis without contrast showed a thick-walled fluid collection measuring 7.9x5.2 x12.0 cm localised to the abdominal wall superficial to the hernia mesh with surrounding fat stranding, consistent with abscess (Fig. 1).
Figure 1. Initial computed tomography (CT) of the abdomen and pelvis showing thick-walled fluid collection (blue arrow) superficial to the hernia mesh (red arrow)
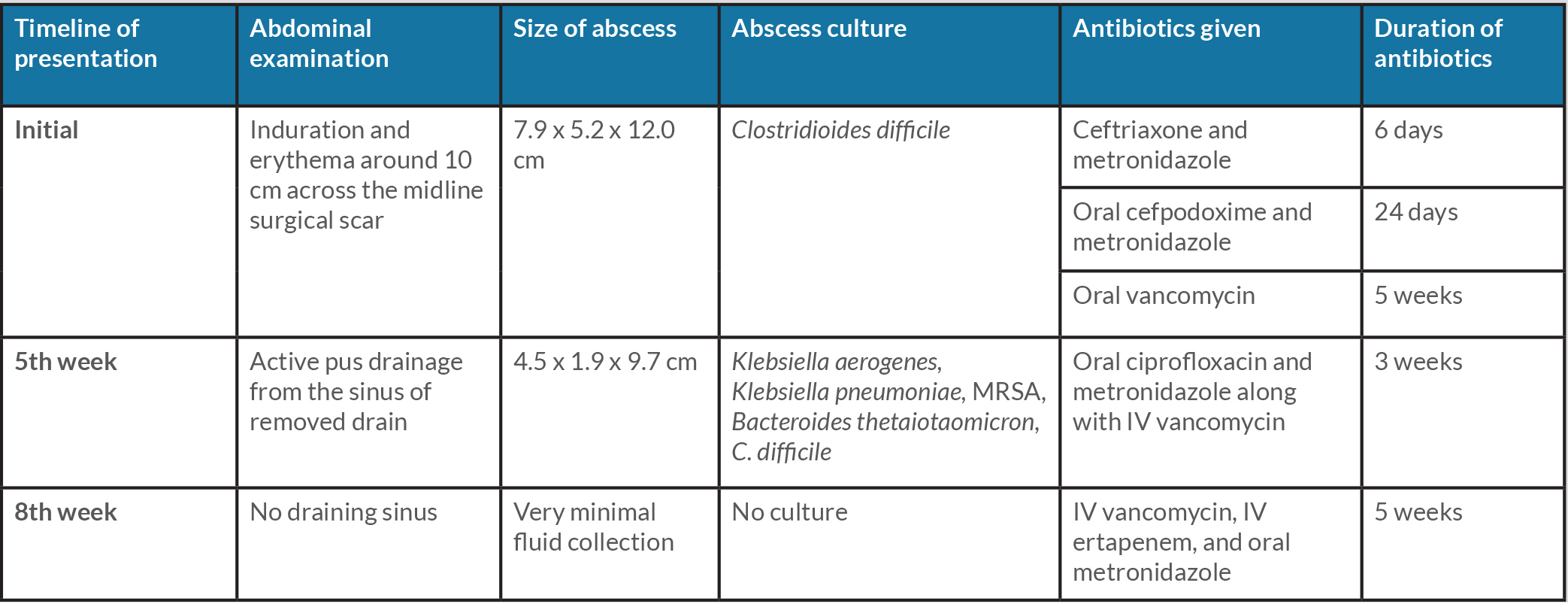
Empiric broad-spectrum intravenous antibiotic therapy (vancomycin, cefepime and metronidazole) was initiated pending results (summary of clinical course is enumerated in Table 1). The abscess was drained under CT imaging guidance with placement of a drainage catheter yielding turbid serosanguinous fluid. The anaerobic culture grew moderate C. difficile while the aerobic culture was negative. Blood cultures were also negative. Stool enzyme immunoassay for C. difficile toxin was negative. The patient subsequently attained haemodynamic stability and vasopressors were weaned. Antibiotics were de-escalated to ceftriaxone, and metronidazole and oral vancomycin. The abscess drainage catheter was exchanged for a larger lumen drainage catheter under fluoroscopic guidance to enhance fluid output. The patient was discharged with the drain in place on oral cefpodoxime, metronidazole, and vancomycin for four weeks further.
The patient was readmitted five weeks following discharge due to complication of the haemodialysis access, which was clotted. Her drainage catheter had been removed in the interim and active drainage of pus was noted from the prior drain tract. Repeat CT of the abdomen and pelvis showed a persistent abdominal wall abscess measuring 4.5 x1.9x9.7 cm. Culture from the drainage grew polymicrobial flora: Klebsiella aerogenes, Klebsiella pneumoniae, methicillin-resistant Staphylococcus aureus, Bacteroides thetaiotaomicron, and C. difficile. Oral vancomycin was stopped while intravenous (IV) vancomycin, cefepime, and metronidazole were resumed. She was transitioned to oral ciprofloxacin and metronidazole along with IV vancomycin on discharge for a total of four weeks.
Table 1. Comprehensive summary of patient’s clinical presentation and treatment given
IV: intravenous, MRSA: methicillin-resistant Staphylococcus aureus
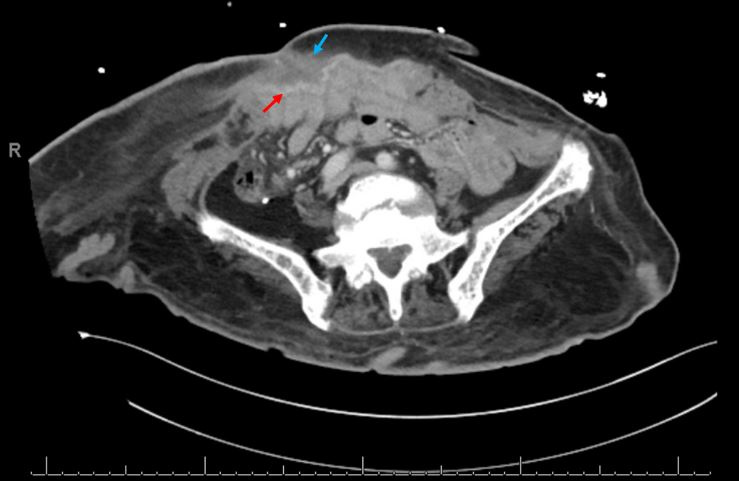
Repeat CT imaging after three weeks of antibiotics demonstrated a small but persistent fluid collection when she was admitted for septic shock features of multifactorial nature, including pneumonia with sputum culture positive for extended spectrum Beta-lactamase producing E. coli (Fig. 2). She was stabilised and was continued on IV vancomycin, IV ertapenem, and oral metronidazole for five weeks. On final follow-up she exhibited clinical improvement with no further discharge from the abdomen. Hence, repeat imaging was not pursued.
Figure 2. Final CT of the abdomen and pelvis showing small residual fluid collection (blue arrow) anterior to the hernia mesh (red arrow)
DISCUSSION
Clostridioides difficile is the most common cause of healthcare-associated infection with an estimated 235,700 cases nationwide in 2017[7]. It is also the most expensive healthcare-associated infection, adding $6.2 billion in healthcare costs annually[8]. Extraintestinal CDI accounts for 0.61% of all cases and most commonly affects the abdominopelvic region. Risk factors include exposure to healthcare settings, antibiotic and proton pump inhibitor (PPI) use, GI surgery (most commonly colon), and malignant tumours[2]. The extraintestinal CDI burden is likely to be underestimated due to the fastidious nature of this anaerobic organism, and reporting of the most predominant coexisting organisms in polymicrobial infection[2]. Our patient had multiple risk factors. First, she suffered an incarcerated parastomal hernia and bowel perforation which was treated with partial ileal resection, and she had an open abdomen with an Abthera device in place for seven days before complete closure of the ostomy and abdomen with mesh. Second, she was in a long-term care facility until a few days before her presentation. Third, she was on chronic PPI therapy and finally, she had diabetes mellitus which is increasingly recognised as a risk factor for CDI in recent studies[9].
The most common pathogenesis of extraintestinal infection is GI perforation faecal spillage. Hence many patients present with polymicrobial infection which includes C. difficile; this may represent spillage of a coloniser rather than an actual pathogen[10-12]. For the same reason not all C. difficile strains isolated at extraintestinal sites are toxigenic[11]. The organism may also, in the absence of perforation, invade the mucosa, translocate into the blood, and disseminate to other sites. Patients with extraintestinal CDI most commonly present within four weeks of the inciting event[2]. Our patient, however, presented with C. difficile abdominal wall abscess almost six months after the initial event. Her case was also unique in that the initial culture revealed monomicrobial growth.
The treatment for extraintestinal CDI is complex and not well defined. This is because the commonly used medication for intestinal CDI such as vancomycin and fidaxomicin have low oral bioavailability, resulting in low therapeutic levels in extraintestinal locations. While metronidazole has good therapeutic levels on oral administration, its use is limited by the toxicity caused by prolonged use[13]. Hence either oral or intravenous metronidazole (if not limited by toxicity), or intravenous vancomycin have been used to successfully treat extraintestinal CDI. Antimicrobial susceptibility testing and tailoring antibiotic regimen is not broadly available in many facilities, and is usually limited to epidemiological purposes in specialised institutes. Rarely is it used to guide specific antibiotic therapy[13]. We (as most institutions) do not have access to sensitivity data on anaerobes including C. difficile. We treated based on the usual resistance patterns with empiric broad-spectrum antibiotics despite the initial monomicrobial culture due to the potential for unrecognised polymicrobial infection. Similar antibiotic regimens were used in other case reports as well[2,14,15]. Our patient subsequently developed proven polymicrobial infection, possibly from translocation of organisms from the exterior through the drainage catheter tract. If an abscess is present as in our case, drainage remains the standard of care in addition to targeted antibiotic therapy[16]. Though she underwent CT-guided drainage of the abscess and extensive antibiotic treatment, the infection persisted. The mesh adjacent to the abscess may have served as continued nidus of infection. Mesh removal was contemplated, but was ultimately deferred given the patient’s comorbidities, and eventual response to medical management alone. We also treated her prophylactically with oral vancomycin to prevent intestinal CDI while on broad-spectrum antibiotics. There is some evidence this strategy is useful for secondary prevention of CDI in certain populations, but not for primary prevention[17-19]. Though the outcome was successful eradication of the infection, the patient’s treatment course was complicated by septic shock, multiple hospital admissions, and prolonged antibiotic therapy.