ABSTRACT
Drug-induced immune haemolytic anaemia (DIIHA) is a rare but serious complication affecting approximately 1 in 1,000,000 patients, but its incidence might be underestimated due to misdiagnosis. Several factors should be considered to ensure an accurate diagnosis, including previous medical history, comorbidities, drug history, the temporal relationship between drug exposure and symptom onset, haemolytic features, and comorbidities in suspected cases. The authors report a case of DIIHA caused by combination chemotherapy with carboplatin and paclitaxel complicated with haeme pigment-induced acute kidney injury.
LEARNING POINTS
- Drug-induced immune haemolytic anaemia (DIIHA) should be suspected in patients with abrupt immune haemolytic anaemia with a temporal relationship between drug exposure and symptom onset.
- The main management of DIIHA consists of urgent discontinuation of the suspected drug and supportive treatment with close monitoring, resulting in a favourable outcome in most cases; the role of corticosteroids in DIIHA remains unclear.
- Haeme pigment-induced acute kidney injury is induced by intravascular haemolysis where urinalysis reveals elevated haemoglobin.
KEYWORDS
Drug-induced immune hemolytic anemia, carboplatin, paclitaxel, heme-induced nephropathy
INTRODUCTION
Drug-related haematological complications may cause a wide variety of abnormalities including haemolytic anaemias, methemoglobinaemia, red cell aplasia, sideroblastic anaemia, megaloblastic anaemia, polycythaemia, aplastic anaemia, leucocytosis, neutropenia, eosinophilia, immune thrombocytopenia, microangiopathic syndromes, hypercoagulability, hypoprothrombinaemia, circulating anticoagulants, myelodysplasia, and acute leukaemia[1]. Drug-induced immune haemolytic anaemia (DIIHA) has been estimated at 1–4 cases per million individuals per year and is believed to account for 16–18% of cases with acquired immune haemolytic anaemia[2].
However, the actual incidence may be greatly underestimated, leading to underdiagnosis. DIIHA is characterized by abrupt immune haemolysis including decreased haemoglobin/haematocrit, increased reticulocytes, elevated indirect bilirubin, elevated lactate dehydrogenase (LDH), decreased haptoglobin and a positive direct agglutination test (DAT) or direct Coombs test, and a temporal relationship between symptom onset and drug exposure[2,3]. Many drugs have been linked to DIIHA, most commonly antibiotics, particularly second- and third-generation cephalosporins, and platinum-based chemotherapies[3,4]. The authors present a case of recurrent haemolytic anaemia following combination therapy with carboplatin and paclitaxel complicated with haeme pigment-induced acute kidney injury.
CASE DESCRIPTION
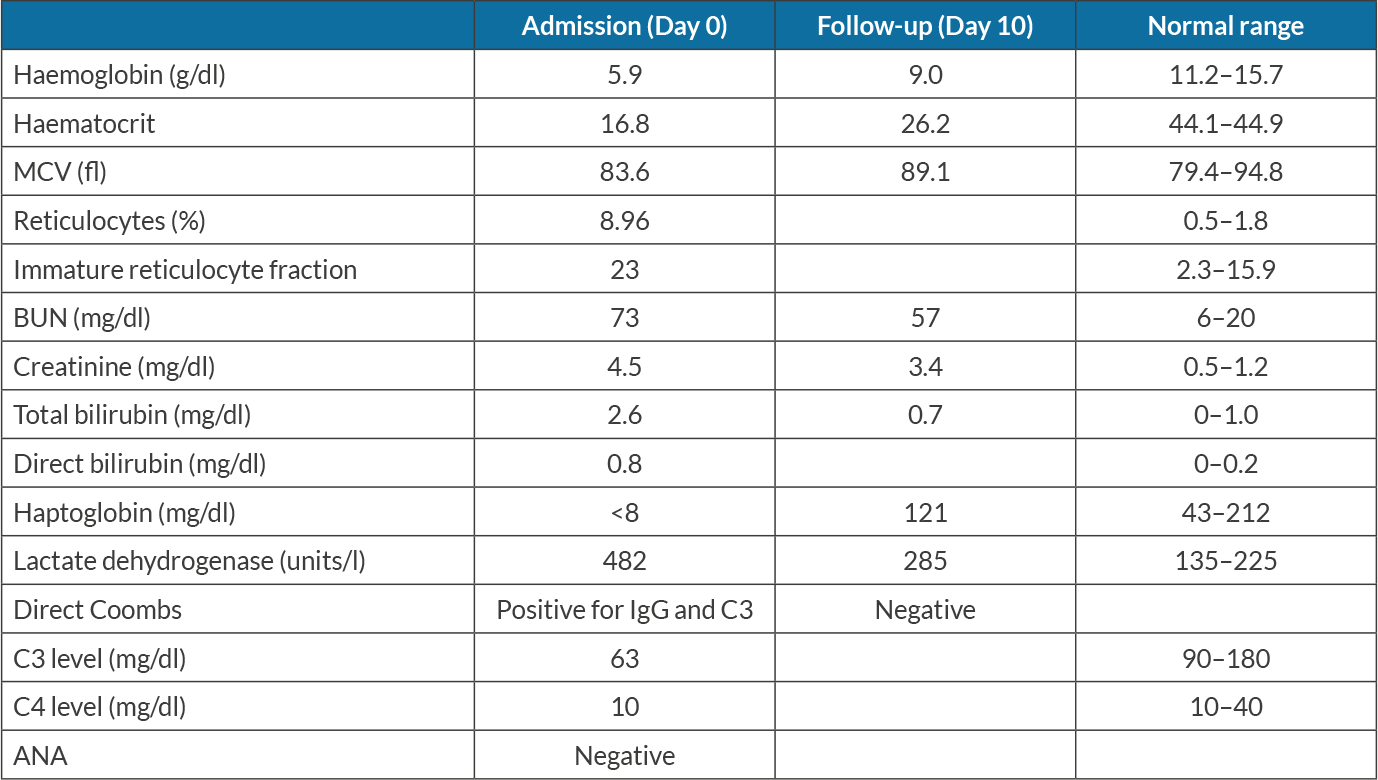
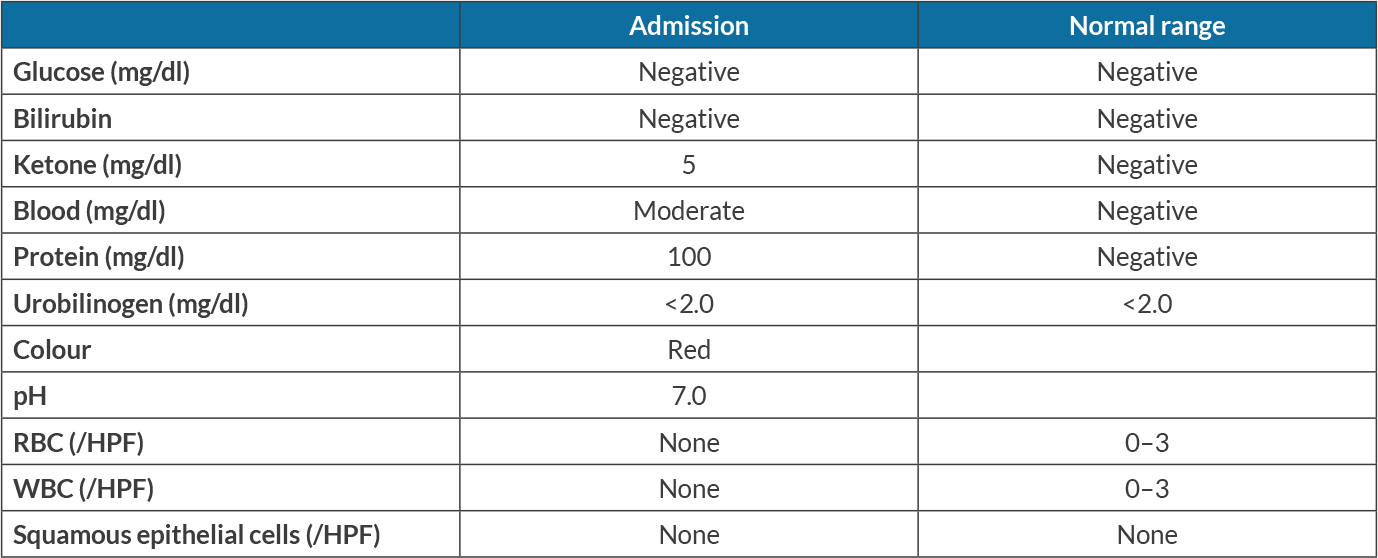
An 82-year-old woman with a medical history of recurrent ovarian carcinoma, hypertension, chemotherapy-induced peripheral neuropathy, osteoarthritis, paroxysmal atrial fibrillation, pulmonary embolism, and urge incontinence presented with a 3-day history of generalized weakness and lethargy. She had noticed that her symptoms started after she had received her scheduled weekly prophylactic combination chemotherapy with carboplatin and paclitaxel. She had experienced multiple episodes of similar symptoms following chemotherapy and required blood transfusions. Upon evaluation, she had pale conjunctiva, and was afebrile, with a blood pressure of 117/68 mmHg, a heart rate of 87 beats per minute, a respiratory rate of 16 per minute, and oxygen saturation of 98% on room air. Initial laboratory results were significant for anaemia with a haemoglobin level of 5.9 g/dl, indirect hyperbilirubinaemia (total/indirect bilirubin 2.6/1.4 mg/dl), elevated LDH (482 units/l), elevated corrected reticulocyte count 3.5%, low haptoglobin (<8 mg/dl) and elevated BUN and creatinine from a baseline creatinine of 0.5 mg/dl, as shown in Table 1. Urinalysis showed moderate blood without red blood cells (RBCs), as shown in Table 2. A peripheral blood smear revealed normocytic normochromic anaemia with elevated red cell distribution width (RDW), scattered schistocytes, lymphopenia, and thrombocytopenia with giant platelets. Rheumatological work-up including anti-nuclear antibody (ANA) and creatinine kinase levels was negative. A direct Coombs test was positive with IgG and C3 coating of RBCs. C3 complement was low (63 mg/dl) with normal C4 complement. Blood transfusions with packed RBCs and intravenous fluids were started. The patient was counselled regarding a bone marrow biopsy but declined any invasive procedures. Based on multidisciplinary discussion with oncology and haematology teams, carboplatin and paclitaxel-induced immune haemolytic anaemia complicated by haeme pigment-induced acute kidney injury was suspected. Supportive management was continued with blood transfusion and IV fluids. Her kidney functions showed moderate improvement with decent urine output not requiring renal replacement therapy. Upon discharge on day 8 of her admission, her haemoglobin was stable with no signs of active haemolysis and normal values of LDH, bilirubin and haptoglobin (Table 1). She had no further anaemia after her prophylactic chemotherapy regimen was discontinued.
DISCUSSION
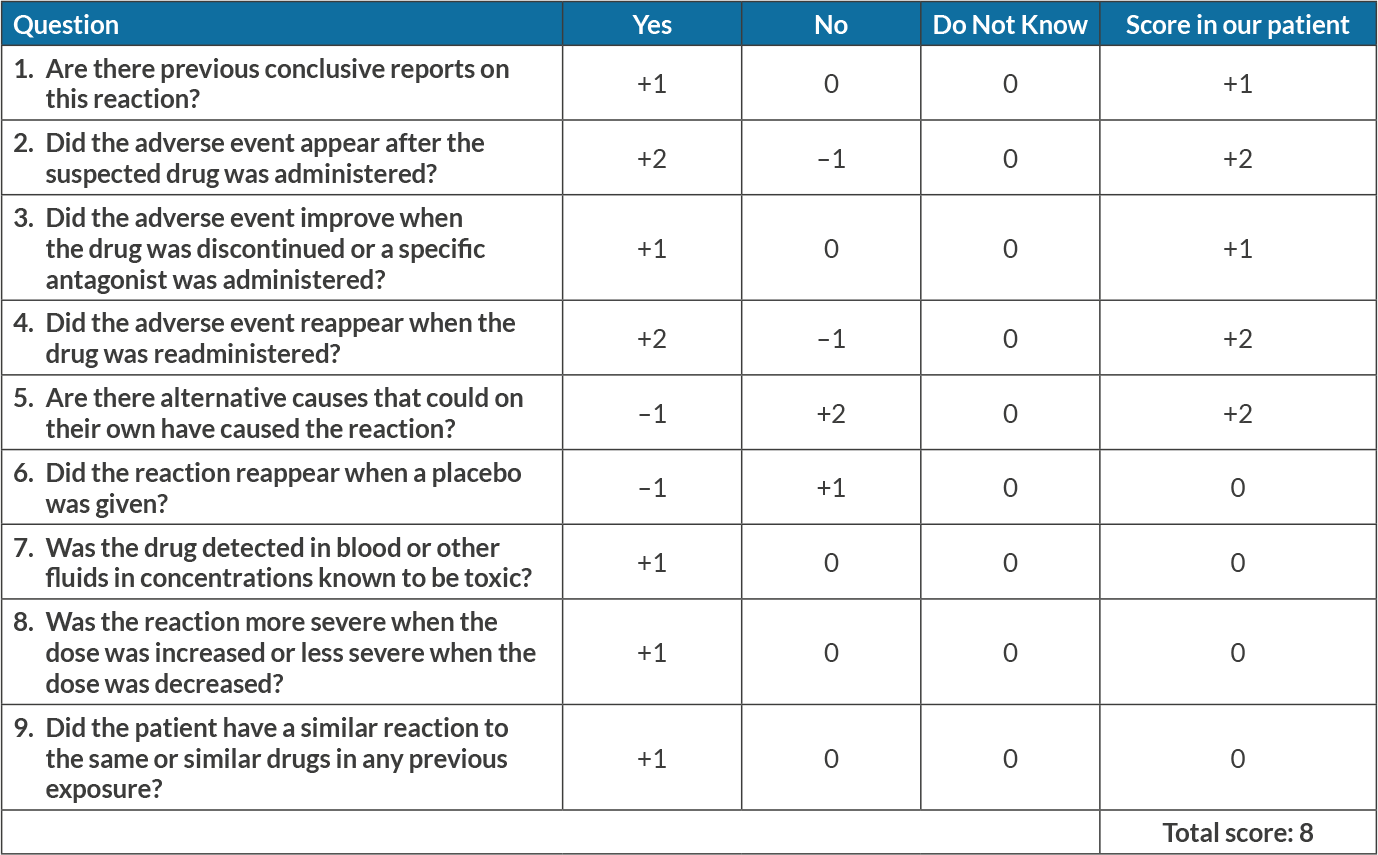
Carboplatin is classified as an alkylating agent and paclitaxel is an antimitotic agent. Combination chemotherapy with carboplatin and paclitaxel is used to treat different types of malignancies including lung, ovarian and cervical cancer. Anaemia in patients treated with carboplatin and paclitaxel is often attributed to their myelosuppressive effect[5]. DIIHA is most often associated with antimicrobials, although platinum-based chemotherapeutics have also been implicated in such adverse reactions[3]. The adverse drug reaction (ADR) probability scale developed by Naranjo et al.[6] (Table 3) indicates our patient had a score of 8, which suggests that carboplatin and paclitaxel were the probable culprit.
Several mechanisms for DIIHA have been proposed including a drug-dependent mechanism in which antibodies can only react in vitro in the presence of a specific drug and which results in haemolytic anaemia through two mechanisms. First, drugs bind to proteins on the RBC membrane, then anti-drug antibodies bind to the drug–RBC membrane complex inducing extravascular haemolysis. Second, combination of anti-drug antibodies results in the formation of immune complexes which subsequently attach to RBC membranes and result in complement activation[7]. A few cases of DIIHA associated with antibodies to carboplatin and paclitaxel have been reported with a DAT positive for anti IgG and anti C3[8,9]. The mainstay of management of DIIHA is discontinuation of the suspected drug. In patients with acute severe DIIHA, fluid resuscitation should be initiated with close monitoring of patient status for prevention of haeme pigment-induced acute kidney injury; intensive care or temporary dialysis may be required. Approximately 55% of DIIHA patients require blood transfusions to maintain adequate haemoglobin levels. The role of glucocorticoids in DIIHA treatment remains unclear[10]. Haeme pigment-induced acute kidney injury can complicate rhabdomyolysis or less commonly intravascular haemolysis where haeme pigment is released from myoglobin or haemoglobin, respectively. Once haemolysis occurs, plasma proteins, such as haptoglobin, bind to free haemoglobin to reduce its deleterious effects. Massive haemoglobin release can occur in the context of intravascular haemolysis. This can overwhelm the binding proteins, so free haemoglobin is filtered into the glomeruli where it degrades, releasing haeme pigment which induces renal injury via vasoconstriction, oxidative stress, direct tubular injury, and tubular obstruction possibly in association with uric acid. Urinalysis typically shows haemoglobinuria and variable proteinuria with the absence of RBCs, while urine microscopy may reveal pigmented, muddy brown, granular casts consistent with acute tubular necrosis[11]. Despite its toxic properties, haeme pigment rarely causes kidney injury in the absence of predisposing conditions, which include volume depletion, metabolic acidosis, and, possibly, mild ischaemia. Treatment is usually supportive with IV fluids and correction of electrolyte abnormalities with favourable prognosis in most cases. Renal replacement therapy might be required for the management of electrolyte abnormalities such as hyperkalaemia, volume overload, severe metabolic acidosis, and uraemic symptoms[12].
Table 3. Naranjo scale: a causality assessment method for assessing all forms of drug-induced adverse events
CONCLUSION
The case highlights that DIIHA should be suspected based on medical history, a temporal relationship between drug exposure and symptoms onset, and haemolytic features. The authors have reported a case of DIIHA caused by combination chemotherapy with carboplatin and paclitaxel.