ABSTRACT
Inferior vena cava (IVC) atresia is a rare congenital vascular malformation. We describe the case of a 20-year-old woman with IVC atresia who presented with a 3-month history of fatigue, oedema of the lower limbs and episodes of lipothymia. Transthoracic echocardiography and cardiac catheterization were performed, revealing interruption of the IVC with circulation through the azygos and hemiazygos system. An abdominal and pelvic computerized tomography (CT) scan confirmed the findings, demonstrating the absence of the IVC below the renal veins. Blood tests did not reveal any relevant results. These findings are consistent with the diagnosis of IVC atresia, a rare condition with no standard treatment. As a surgical approach was not possible, pharmacological measures were implemented for primary prevention of possible thrombotic events.
LEARNING POINTS
- Inferior vena cava atresia is a rare vascular malformation.
- The clinical presentation is non-specific: most diagnoses are made after a thrombotic event or as an incidental finding on imaging.
- Consensus on definitive treatment is lacking, but the increased thrombotic risk warrants primary prevention of thrombosis.
KEYWORDS
Inferior vena cava, vascular malformations, collateral circulation
CASE DESCRIPTION
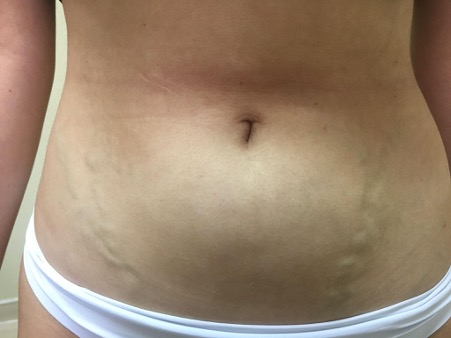
A 20-year-old Caucasian woman without a relevant medical history, presented to an outpatient clinic with complaints of fatigue, lower limb oedema and several episodes of lipothymia that had started 3 months earlier. The patient was not on any medication and had no family history of venous thromboembolism, coagulopathies or congenital pathologies. On physical examination, exuberant abdominal collateral circulation in the lower abdominal quadrants was noted (Fig. 1).
There were no changes on electrocardiography or chest radiography. However, transthoracic echocardiography revealed an intrahepatic vessel with a similar trajectory to the IVC draining to the right cardiac atrium, but without extrahepatic continuity. The abdominal and pelvic CT scan revealed collateral circulation in the abdominal and thoracic walls, with abdominal and pelvic venous drainage through the azygos and hemiazygos veins, converging to the superior vena cava. No iliac veins or IVC were identified below the renal veins (Figs. 2–4). These findings were also confirmed with cardiac catheterization. There were no suspicious serological findings, with functional and molecular thrombophilia studies (anticardiolipin antibody, beta 2-glycoprotein, lupus anticoagulant, protein C and S, factor II and V Leiden) being negative.
After a multidisciplinary team meeting, it was concluded that surgical correction was not feasible, and primary prevention measures should be implemented (lower limb compression stockings and oral anticoagulants) to decrease thrombotic risk.
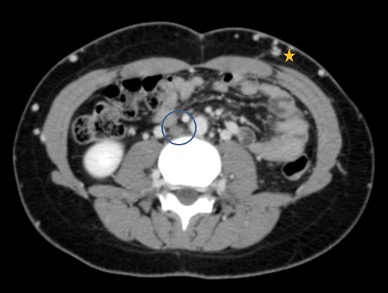
Figure 2. Thoraco-abdomino-pelvic computed tomography (CT) in the portal venous phase. The inferior vena cava (IVC) is not identified in its normal position, lateral to the abdominal aorta (circle). Multiple collateral vessels are observed in the abdominal wall (star)
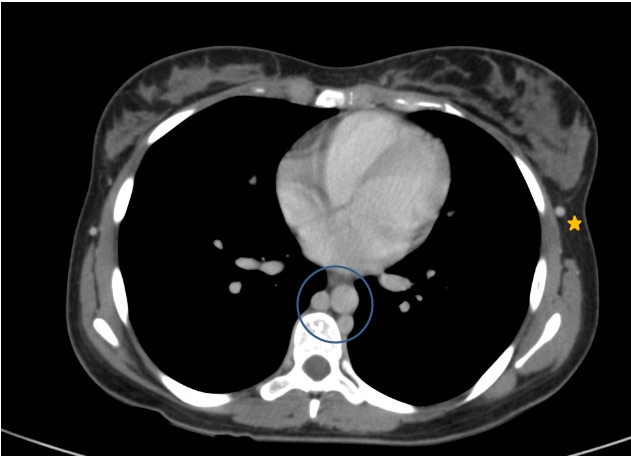
Figure 3. Thoracic CT images in the portal venous phase show that abdominal venous drainage occurs through the azygos and hemiazygos veins (circle). The thoraco-epigastric veins are engorged up to their confluence with the axillary veins (star)
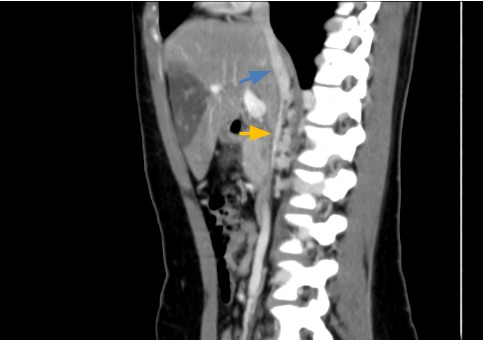
Figure 4. Sagittal CT images show a short segment, mainly intrahepatic, of the IVC. It begins after the confluence of the renal veins, where it has a filiform calibre (orange arrow), later receiving collateral vessels up to an intrahepatic portion (blue arrow), which has the usual calibre and course, draining to the right atrium
DISCUSSION
Anatomical variations of the IVC are secondary to several changes that occur during embryogenesis [1, 2]. The IVC is formed between the fifth and seventh weeks of gestation from the fusion of three pairs of veins (supracardinal, subcardinal and posterior cardinal), which may give rise to numerous anatomical variations [2]. Atresia of the IVC can be characterized as partial or complete non-development of the vein [1]. There is insufficient knowledge on the incidence of congenital IVC agenesis [1]. Perinatally acquired venous thrombotic events and hereditary thrombophilias are possible causes [1].
For this reason, all patients diagnosed with IVC atresia should undergo a blood hypercoagulability study [1]. The remaining organs and blood vessels of the chest and abdomen usually have no congenital anomalies, and coexisting congenital cardiac anomaly is rare [2, 3].
Most patients are asymptomatic due to the compensatory development of collateral circulation in the azygos and hemiazygos veins [1–3]. This collateral circulation drains into the superior vena cava, which in turn ends in the right auricle [4]. However, there are various non-specific signs and symptoms, among which lower limb oedema, abdominal pain and lower limb varices are the most common [1–3]. Prevalence is higher in males [3].
Patients with IVC atresia are prone to develop lower limb deep vein thrombosis, secondary to stasis and increased venous pressure, and pulmonary thromboembolism [1, 3, 4].
The diagnosis is made from the non-identification on imaging of the IVC in its normal position and by the presence of venous collateral vessels, namely prominent azygos and hemiazygos veins [1, 2]. Computerized tomography and magnetic resonance imaging are the standard imaging methods for diagnosis [2, 3].
Due to the rarity of this pathology, consensus on the best therapeutic approach is lacking and further studies are needed to support patient stratification and treatment [5]. Therapeutic strategies are currently based on medical and surgical approaches, such as anticoagulation and endovascular reconstruction and bypass graft surgery [5].