ABSTRACT
According to the modified World Health Organization (WHO) classification, mechanical valves pose a high maternal risk and complications for pregnant women with heart disease. Left atrial appendage aneurysm (LAAA) is a rare condition that can manifest clinically in several ways or remain silent for a long time and can be either congenital or acquired. We present the case of a pregnant woman who had a LAAA discovered several years after her last mitral valve replacement.
LEARNING POINTS
- Left atrial appendage aneurysm is a rare entity and, in most cases, is congenital due to poor myocardial contractility of dysplastic pectinate muscles.
- Clinical manifestations range from an asymptomatic course with an incidental finding on echocardiography up to serious sequelae such as cardioembolic manifestations.
- The treatment approach includes a conservative strategy using anticoagulation and a surgical strategy with aneurysmectomy.
KEYWORDS
Left atrium, left atrial appendage aneurysm, mechanical mitral prosthesis
INTRODUCTION
Almost 150 cases of left atrial appendage aneurysm (LAAA) have been reported in the literature over the last 60 years. Early diagnosis and recognition, followed by treatment, may prevent serious complications that could occur if the condition remains undiagnosed. Thromboembolic events could be the first sign of LAAA. We describe the case of a pregnant woman who presented with mild shortness of breath and a history of multiple mitral valve prosthesis replacements and was discovered to have a huge acquired LAAA.
CASE DESCRIPTION
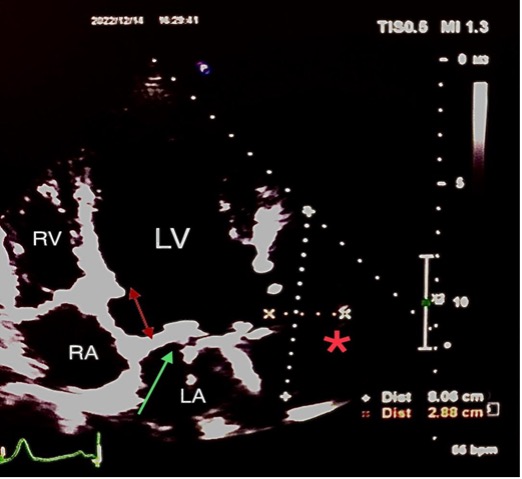
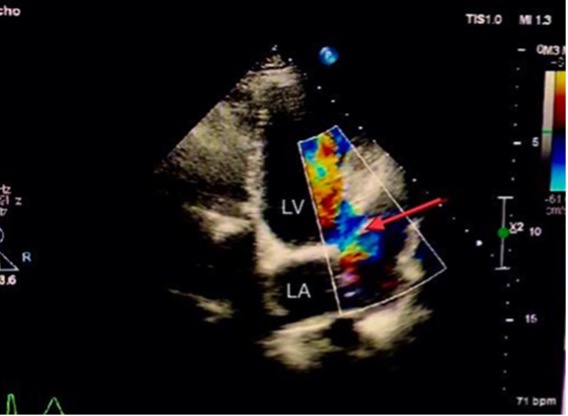
A 38-year-old woman presented at 8 weeks of pregnancy (gravida 3, para 2) with a history of rheumatic heart disease and severe mitral stenosis since childhood which had been treated in 2004 with bioprosthetic mitral valve replacement. She had later delivered two normal infants. She had remained asymptomatic until 2016, when she had presented with progressive shortness of breath. Echocardiography showed severe degenerative changes in the bioprosthetic mitral valve. The decision was to perform a second mitral valve replacement with a metallic valve. She underwent metallic mitral valve replacement with left atrial appendage ligation. A few days after the operation, she went into severe respiratory distress and refractory heart failure, and a severe paraprosthetic leak was diagnosed, requiring redo surgery. During the redo surgery, supra-annular mitral valve replacement with a 29 mm CarboMedics prosthetic valve was performed due to poor tissue support at the level of the mitral annulus. The new prosthetic valve was sutured to the left atrial wall approximately 10 mm above the original annulus, including the coumadin ridge, to secure firm suturing for valve implantation. The supra-annular position of the prosthesis led to the inclusion of the ligated left atrial appendage into the left ventricular (LV) cavity without being involved in circulation because it was ligated. The patient recovered slowly from the redo surgery and was discharged home on therapeutic INR with advice to use contraception since she is considered to be at high maternal cardiovascular risk. There was no follow-up with echocardiography during the next few years, and her follow-up was restricted to the adjustment of INR to the therapeutic level. In 2022, the patient became pregnant, and at 8 weeks of pregnancy presented for consultation and complained of mild shortness of breath. She was on warfarin 5 mg to maintain a therapeutic INR for her metallic mitral valve. Physical examination was remarkable for a metallic sound with a grade 2/5 systolic murmur. Her electrocardiogram showed sinus rhythm. Transthoracic echocardiography (TTE) showed a mildly dilated LV with good systolic function. The bileaflet metallic valve was noted in the supra-annular position with normally opening discs. No significant prosthetic or paraprosthetic regurgitation was detected on the TTE views. A myocardial defect located below the lateral annulus of the mitral valve near the end of the anterolateral wall of the LV measuring 1.5 cm and connected to a huge cavity measuring 3×8 cm was noted (Fig. 1). Colour Doppler showed flow into the cavity and back to the LV (Fig. 2 and Video 1).
Figure 1. Two-dimensional echocardiographic scan showing the supra-annular location of the mechanical mitral valve (red double arrow and green arrow). A defect noted at the level of the native lateral mitral annulus near the end of the anterolateral wall of the LV measuring 1.5 cm and connected to a huge cavity (red star) measuring 2.9×8 cm raised the suspicion of a left atrial appendage aneurysm. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle
Figure 2. Doppler colour flow showed flow with a to-and-fro pattern between the LV through the defect and aneurysmal cavity of the left atrial appendage (red arrow). LA, left atrium; LV, left ventricle
Video 1
Video 1. Colour Doppler showing flow into the cavity and back to the left ventricle
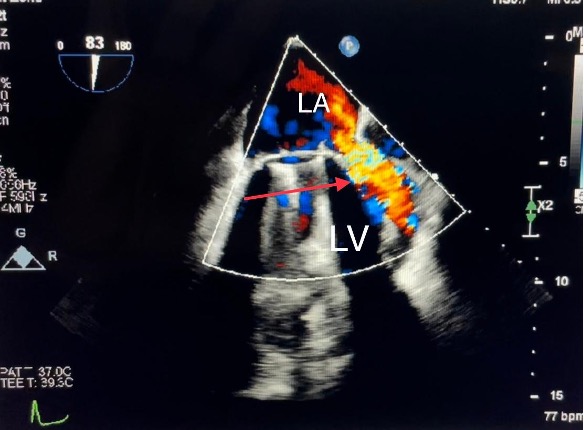
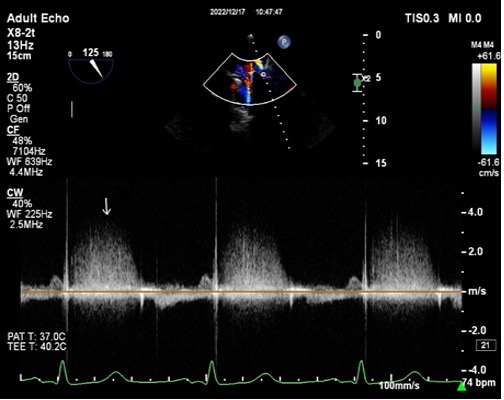
The supra-annular position of the mitral prosthesis, with the subsequent inclusion of the ligated left atrial appendage into the LV cavity, led to the suspicion that the large cavity was a LAAA. The cavity was free of masses or thrombi. Transesophageal echocardiography showed the supra-annular position of the metallic valve with normal movement of the occluders and a normal flow gradient across the prosthesis. However, on colour Doppler, a significant paravalvular leak was detected at the sewing ring’s insertion into the coumadin ridge with an accelerated systolic flow mainly directed into the left upper pulmonary vein (Fig. 3 and Video 2). The velocity of the paravalvular leak flow was 3 m/sec (Fig. 4). Unfortunately, the LAAA was not properly visualized because of artifacts from the prosthesis. Compared with the previous echocardiography study done in 2016, the large cavity was a new finding. The laboratory findings showed no evidence of anaemia or haemolysis. The INR level was in the therapeutic range.
Figure 3. Transesophageal echocardiography, two-chamber view. Doppler colour flow showed a significant paravalvular leak detected at the point of insertion of the sewing ring into the coumadin ridge with an accelerated systolic flow (red arrow). LA, left atrium; LV, left ventricle
Figure 4. Colour Doppler flow showing an accelerated flow with approximately 3 m/sec (white arrow)
Video 2
Video 2. Colour Doppler showing a significant paravalvular leak
A possible explanation for the acquired LAAA was the gradual dehiscence of sutures of the left atrial appendage over the years and the fact that it was subjected to the high pressure of the LV cavity with subsequent blood flow from the LV into the left atrial appendage, which led to a gradual increase in the size of the left atrial appendage. In addition, the therapeutic anticoagulation the patient was on probably prevented the formation of a thrombus inside the left atrial appendage. On the other hand, the stretching of the left atrium and the increase in LA tension over the years weakened the support of the sewing ring in the insertion point near the coumadin ridge, which gradually developed into paravalvular leakage. Another imaging modality was not employed due to pregnancy.
The patient’s mechanical valve placed her in a high maternal cardiovascular risk group, but because she wanted to continue her pregnancy and there was no evidence of significant haemodynamic consequences, in addition to the absence of haemolysis and anaemia, the decision was made to closely follow her up conservatively.
DISCUSSION
The first description of a congenital LAAA was published in 1960 under the title ‘The case of the giant dog ear’ in a 47-year-old male patient who was treated with resection[1]. LAAA is considered a rare pathology, and approximately 150 cases only have been reported since it was first described in the literature[2]. The clinical manifestations range widely from a totally asymptomatic course up to life-threatening complications, with one-third of cases discovered incidentally on echocardiography and another third manifesting different symptoms and signs after several decades[3]. LAAA can be either congenital in 90% of cases or acquired due to a condition leading to elevated left atrial pressure, including different mitral valve pathologies[4]. The proposed pathophysiological mechanism of left atrial appendage growth includes poor myocardial contractility of dysplastic pectinate muscles that can produce either local or diffuse outpouching and enlargement of the left atrial appendage with its subsequent conversion to a reservoir instead of a pump[5]. Histopathology of both types of LAAA showed fibrosis of the endocardium and myocardium, which explains the appearance of arrhythmias as a manifestation and palpitation as the most common complaint[6]. A left atrial appendage over 5 cm is considered huge, and its growth can increase with patient age and with any condition raising pressure inside the LA. This explains why the patient's symptoms appeared mostly in their fourth decade. Females tend to have a slightly higher percentage of LAAA than males. The growth in the size of a left atrial appendage can produce a compression effect on adjacent cardiac structure; however, other manifestations, such as atrial arrhythmias, dyspnoea and thromboembolic events, may also present[7].
The structural remodelling of the left atrial appendage leads to different rhythm disturbances, such as atrial fibrillation and supraventricular tachycardia, which can be the first manifestation of LAAA[8]. The loss of contractile function of the left atrial appendage due to aneurysmal enlargement and dilatation will cause blood stasis and subsequent formation of thrombi in the aneurysmal cavity, which significantly increases the possibility of thromboembolic events. Li et al. reported a case of acute massive cerebral infarction and atrial fibrillation in a 35-year-old male patient with giant congenital LAAA. In this case, surgical resection was the only effective treatment to restore and maintain sinus rhythm[9]. Anatomically, LAAA can be classified as extrapericardial or intrapericardial[11].
In most reported cases, LAAA is considered an isolated congenital pathology which is only very rarely associated with other cardiac anomalies such as atrial or ventricular septal defects[10]. In a review of the literature, Wang et al. noted that the main complaint in symptomatic patients with LAAA was palpitations, while shortness of breath and chest pain were less frequent[11]. Since the symptoms of left atrial appendage overlap with several other cardiovascular pathologies, imaging is essential for diagnosis and subsequent treatment[12]. Although chest x-rays in most reviewed patients were abnormal, these abnormalities were not specific. If LAAA is suspected, TTE plays an important role in detecting a large saccular echo-free structure laterally to the LV, while colour Doppler is used to visualize blood flow between the cavity and left atrium, in addition to excluding other cardiac pathologies. In some cases, contrast-enhanced echocardiography can be helpful to identify the connection between the LA and the aneurysmal cavity, and particularly to rule out the presence of a thrombus that may change the therapeutic options[13]. If the picture on TTE is ambiguous, then transesophageal echocardiography is considered mandatory[14].
Cardiac computed tomography (CT) and magnetic resonance imaging (MRI) can visualize LAAA and are considered useful imaging modalities for diagnosing LAAA and ruling out other differential diagnoses, in addition to assessing the surrounding structures for the possible compressive effects of LAAA. MRI has the highest temporal resolution. Furthermore, Wang et al. reported that 31 of 34 cases of LAAA were identified by cardiac MRI[11].
Currently, there is no widely accepted consensus regarding the management of LAAA. However, to prevent severe complications and consequences once the diagnosis is established, early surgical intervention seems to be an effective strategy[3, 7]. On the other hand, a few reports have suggested a more conservative strategy, including no surgical intervention or late surgery, which could be a more suitable alternative for patients with no or mild symptoms[15]. The conservative strategy includes close monitoring; in some cases, anticoagulation may be considered[15]. Aneurysmectomy with or without cardiopulmonary bypass is considered the surgical treatment of choice, and median sternotomy aided by cardiopulmonary bypass is the most commonly reported operative approach.
In our case, two factors contributed to the formation of the huge aneurysmal cavity of the left atrial appendage. The first factor was the supra-annular position of the metallic mitral prosthesis inserted during the last redo surgery that resulted in the inclusion of a left atrial appendage into the LV cavity. The second factor was the possible incomplete ligation and gradual dehiscence of sutures of the left atrial appendage, which created communication between the left atrial appendage and the high-pressure LV cavity. The combination of both factors resulted in gradual enlargement of the left atrial appendage and the formation of a huge aneurysmal cavity. Fortunately, the patient was on therapeutic anticoagulation as indicated for the metallic valve, which probably prevented thrombi formation and thromboembolic events. The patient’s pregnancy precluded us from performing further imaging.
Our case demonstrated an acquired iatrogenic type of LAAA treated conservatively by close monitoring and continuation of anticoagulation.
CONCLUSION
LAAA is rare and most cases are congenital. The combination of pregnancy and acquired LAAA is extremely rare.