ABSTRACT
Organizing pneumonia (OP) is a form of interstitial lung disease that develops in response to acute lung injury. SARS-CoV-2 causes a wide range of lung and extrapulmonary disease, but there are few data suggesting an association between COVID-19 and OP. We describe a patient with COVID-19 pneumonia who developed severe progressive OP with significant morbidity.
LEARNING POINTS
- COVID-19 pneumonia is one of the secondary causes of organizing pneumonia (OP).
- Early initiation of steroids in OP is associated with improvement in symptoms and prognosis.
- A prolonged course of steroids may be needed in COVID-induced OP.
KEYWORDS
Pneumonia, organizing pneumonia, COVID-19, SARS-CoV-2, coronavirus
INTRODUCTION
Organizing pneumonia (OP) is a rare subtype of interstitial lung disease that develops in response to acute lung injury[1]. Formerly known as bronchiolitis obliterans organizing pneumonia (BOOP), OP is characterized by the formation and deposition of granulation tissue, termed Masson bodies, in alveoli and bronchioles, leading to the disruption of proper gas exchange and subsequent respiratory failure. The development of OP occurs in stages, beginning with damage to type I pneumocytes and alveolar basal lamina resulting in the leakage of pro-coagulative proteins, subsequent fibrin deposition, and the organization of fibroblasts, myofibroblasts and collagen into a connective tissue matrix within the distal respiratory tract[2–4].
There are two types of OP: cryptogenic and secondary. Cryptogenic OP is idiopathic in nature and considered a diagnosis of exclusion after all other known causes of OP have been ruled out[4]. Secondary OP is associated with autoimmune diseases, including rheumatoid arthritis, haematological malignancies, certain medications, and most notably, infection[5]. Known viral triggers of secondary OP include influenza virus, parainfluenza virus, cytomegalovirus, adenovirus and human immunodeficiency virus[5,6]. To date, there have been limited reports of patients developing secondary OP following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel zoonotic virus that causes coronavirus disease 2019 (COVID-19).
CASE DESCRIPTION
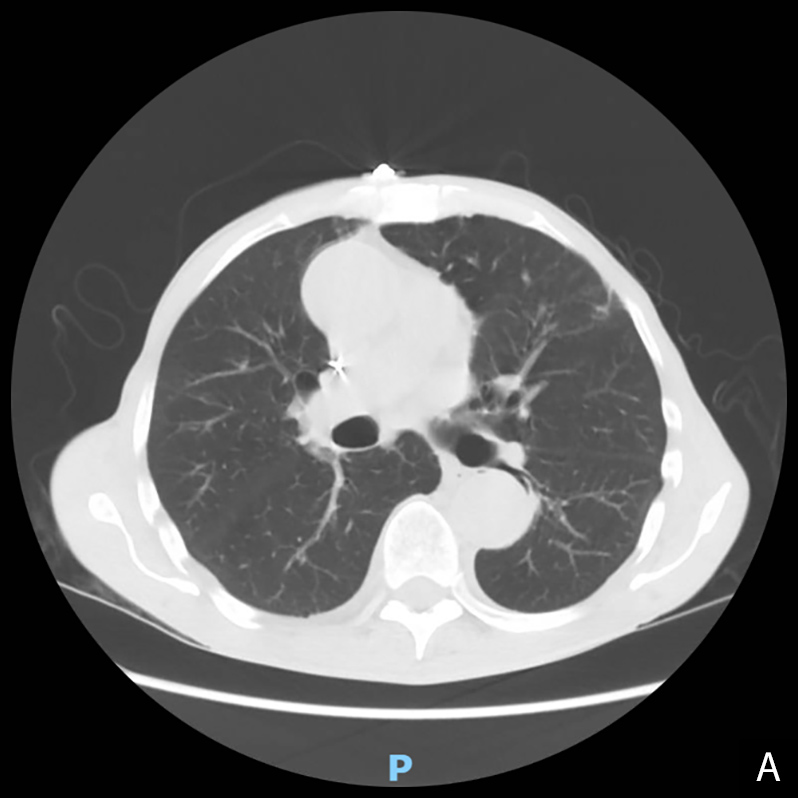
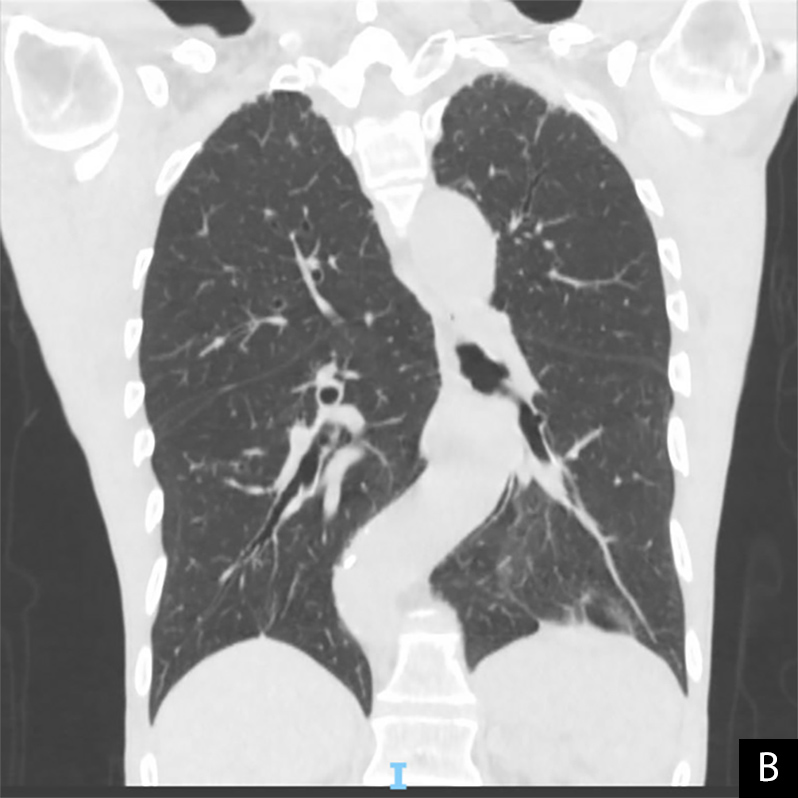
A 73-year-old man with a past medical history of chronic kidney disease stage III, paroxysmal atrial fibrillation and hypertension presented to the hospital with fatigue, exertional dyspnoea, poor appetite and unintentional weight loss. He tested positive for SARS-CoV-2 at admission. He had received both COVID-19 vaccines earlier that year. The initial chest x-ray showed a left upper lobe nodular opacity stable from previous CT chest imaging and no acute cardiopulmonary process. CT chest imaging revealed a 1.0×1.0 cm and nodular-like density in the lingula with dense central calcification and 1 to 2 mm scattered pulmonary nodules, both grossly stable from previous imaging (Fig. 1A,B). The patient was discharged home on day 2 with improved shortness of breath and heart rate.
Figure 1. Non-contrast chest CT at initial hospital admission showing paraseptal emphysematous changes, stable bi-apical scarring, bandlike bibasilar opacities, and stable scattered 1–2 mm pulmonary nodules. A: axial plane, B: coronal plane
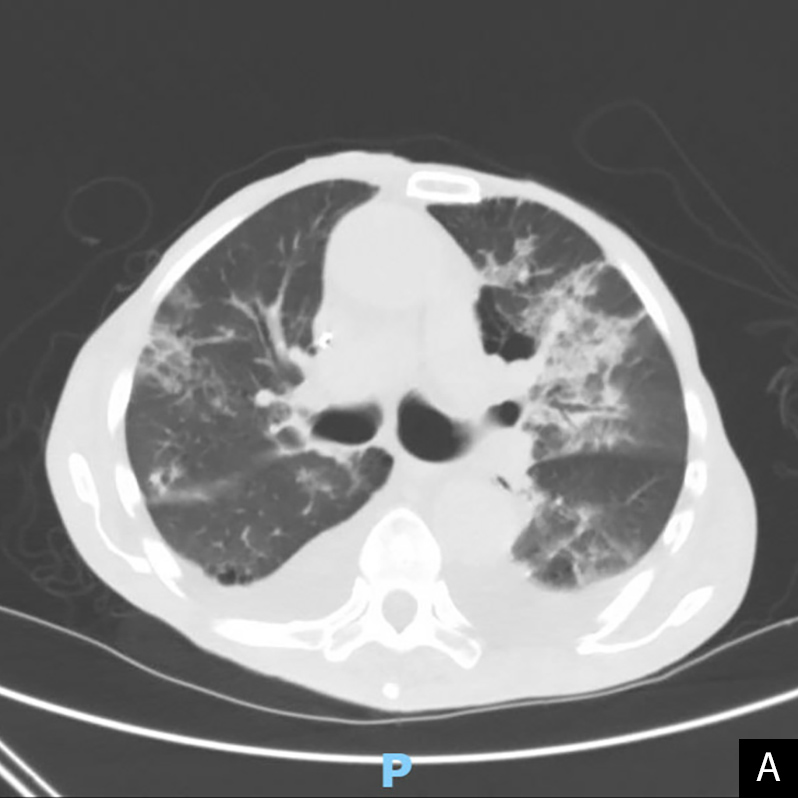
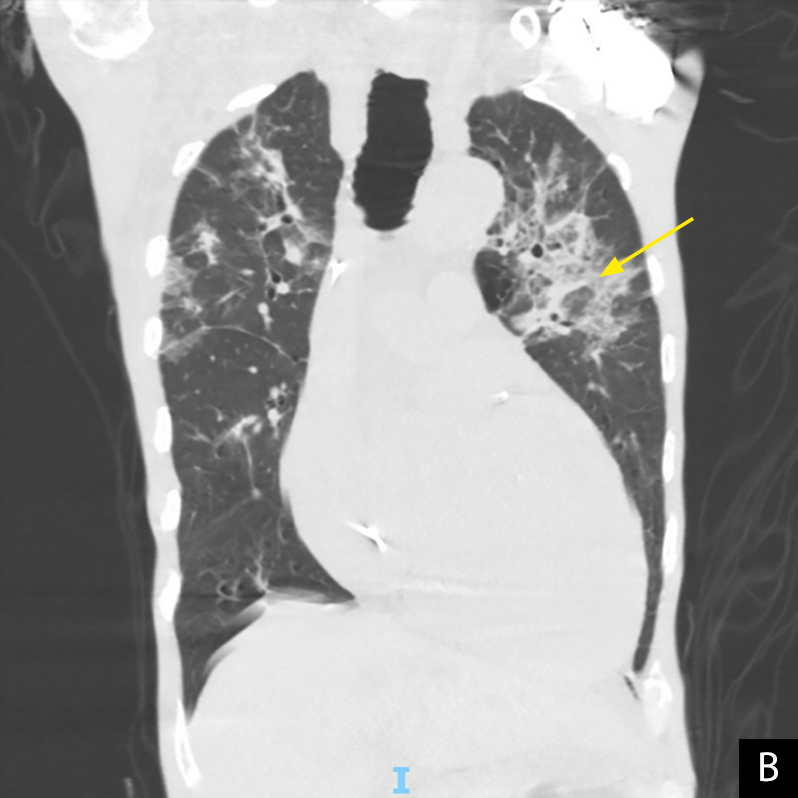
One month later, the patient underwent a lung biopsy for his chest CT findings. Biopsy results revealed non-necrotizing granulomatous inflammation and the diagnosis of cryptogenic OP was made. The patient returned 2 months later with complaints of regressive chronic shortness of breath and progressive weight loss thought to be post-COVID OP, and was started on prednisone 60 mg/day. He showed marked improvement and was discharged home on day 4, but returned 3 days later with worsening weight loss, failure to thrive, and severe back pain. At admission, he was afebrile with a respiratory rate of 12 breaths/min, and SpO2 97% on room air. Laboratory results revealed a WBC count of 10.1, BUN 48, Cr 1.7, BNP 160, and a negative SARS-CoV-2 RT-PCR test. He was noted to have worsening opacities on chest x-ray. Chest CT revealed bilateral ground-glass opacities predominantly in the periphery of the upper lobes, scattered pulmonary nodules stable from previous CT, and small-to-moderate bilateral pulmonary effusions (Fig. 2A,B). He was started on IV antibiotics for possible bacterial pneumonia and re-started on prednisone 60 mg/day. During his hospital course, the patient continued to have significant dyspnoea despite being on prednisone and later developed new-onset altered mental status affecting oxygen compliance. Pulmonology and palliative care were consulted, and the patient was transitioned to comfort care. He later deteriorated and died 2 days after discharge.
Figure 2. Non-contrast chest CT, 4 days after re-admission showing ground-glass opacities predominantly in the bilateral upper lobes with predominant peripheral, subpleural distribution, stable bi-apical scarring, stable scattered 1–2 mm pulmonary nodules, and a possible reverse halo sign (yellow arrow). A: axial plane, B: coronal plane
DISCUSSION
COVID-19 is caused by infection by SARS-CoV-2 and manifests with a wide variety of symptoms, ranging from constitutional symptoms, such as fever and myalgias, to more localized manifestations, such as dyspnoea and cough[7]. SARS-CoV-2 mediates its effects through its interaction with angiotensin-converting enzyme 2 (ACE2) receptors found throughout the body, with the highest density of ACE2 receptors being found on the surface of alveolar epithelial cells. Upon invasion of these epithelial cells, two simultaneous events occur: viral replication within the host cell leading to increased viral load and subsequent invasion of neighbouring alveolar cells, and the secretion of pro-inflammatory cytokines, stimulating the migration of leukocytes to the site of infection[8]. Although the exact mechanism of pathogenesis is not well understood, it is hypothesized that dysregulation of the adaptive immune system following activation of the innate system paradoxically leads to hyperinflammatory immune activation and simultaneous systemic immune suppression[9].
Given SARS-CoV-2 predisposition to target alveolar epithelial cells, most patients typically present with symptoms of an upper respiratory infection; however, more severe complications including the development of acute respiratory distress syndrome (ARDS), pulmonary fibrosis with worsening respiratory failure, shock, multiple organ dysfunction syndrome (MODS) and death have been reported[10]. Patients who undergo resolution of their acute viral illness may still experience chronic health complications for weeks or months afterwards. This state of chronic immunosuppression is termed long COVID[11]. These patients can be at risk of developing permanent pulmonary function impairment, kidney injury, metabolic derangements, persistent dyspnoea, chest pain and myalgias, decreasing their overall quality of life and predisposing them to potential comorbidities.
Our patient complained of persistent dyspnoea, weight loss and failure to thrive for 3 months following his initial COVID-19 illness, despite receiving both vaccine doses earlier that year. He was diagnosed with cryptogenic OP at his scheduled lung biopsy 1 month after his initial presentation and continued to have worsening respiratory and constitutional symptoms. Comparison of chest CT images from his first and last admissions revealed bilateral ground-glass opacities with predominant peripheral, subpleural distribution, and a possible atoll or reverse halo sign, all characteristic of OP[12,13].
The use of corticosteroids has been found to improve overall patient outcomes and decrease the rate of readmission in patients with secondary OP[6,13]. Current recommendations for the management of OP in the setting of COVID-19 infection include the use of prednisone for at least 4–8 weeks, but higher rates of recovery have been reported with the use of steroid therapy upwards of 6–12 months after symptom onset given the risk of possible relapse[2,13]. The RECOVERY trial investigated the use of methylprednisolone in COVID-19 patients and found an overall decrease in the 28-day mortality rate in patients who received invasive mechanical ventilation or received supplemental oxygen, not on mechanical ventilation[14]. It is important to note that, while most studies report symptomatic and radiological improvement following prolonged use of corticosteroids in patients with COVID-19 infection and secondary OP, there have been some reports suggesting that not all patients respond to steroid therapy[12,15].
Our patient was started on 60 mg/day of prednisone on both admissions and showed initial improvement of his pulmonary symptoms following treatment. Throughout his last hospital course, however, our patient began to deteriorate, developing delirium and agitation, and increased oxygen requirement. Per the patient’s healthcare proxy, his code status was changed to Comfort care and was discharged to hospice care where he died.
CONCLUSION
OP can be one of the many complications associated with infection by SARS-CoV-2 and should be considered as a differential diagnosis when patients presenting with ongoing or relapsing respiratory symptoms are being cared for. Given the limited documentation of secondary OP following COVID-19, it is important to bring attention to it to ensure prompt diagnosis and treatment. Managing these patients with corticosteroids has been found to be overall beneficial in treating secondary OP but requires a prolonged treatment course to be considered effective in preventing relapse.