ABSTRACT
Introduction: High altitude illness is a complication of rapid ascent above 2,500 m elevation. Ventilatory, circulatory and haematological adjustments, known as acclimatization, occur to maintain adequate delivery of oxygen. Although (non-)pharmaceutical strategies that modulate ventilation and circulation have long been accepted, the haematological approach has not.
Case description: This report describes the application of a comprehensive strategy, including prior pre-acclimatization using an erythropoiesis-stimulating agent (ESA), in two healthy subjects ascending from sea level to 6,268 m. Following ESA administration 30 days prior to ascent, the subjects had a cumulative haemoglobin rise of 7.1% and 11.9%, respectively. Both subjects experienced minimal symptoms during four incremental ascents to the final altitude and no adverse events occurred.
Discussion: This report has limited external validity, lacking both a sample size and controls, but can serve as practical exploration of the concept. Administration of an ESA may be a safe and useful pre-acclimatization strategy but cannot be recommended based on current evidence. More comprehensive research is needed.
LEARNING POINTS
- High altitude illness (HAI) is a debilitating syndrome with potentially lethal consequences caused by ascent to a hypobaric atmosphere without acclimatization.
- Pharmacological strategies aimed at increasing oxygen delivery may be used to prevent and treat HAI.
- Administration of an erythropoiesis-stimulating agent may be a safe and useful pre-acclimatization strategy but cannot be recommended based on current evidence alone.
KEYWORDS
High altitude illness, erythropoiesis-stimulating agent, acclimatization
INTRODUCTION
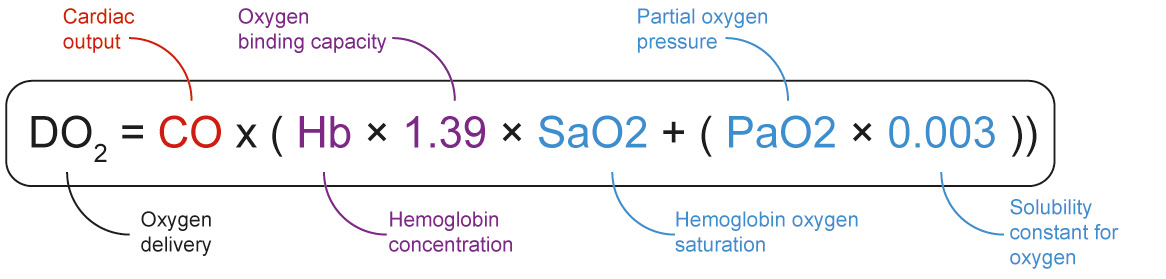
High altitude illness (HAI) occurs when (the rate of) ascent outpaces an individual's ability to physiologically adjust, or acclimatize, to a hypobaric-hypoxic challenge. The three large adjustments are ventilatory, circulatory and haematological – corresponding to components of the oxygen delivery formula shown in Fig. 1. Haematological adaptations can maintain arterial oxygen content up to about 7,100 m of altitude[1]. This adjustment occurs as altitude-related hypoxia induces erythropoiesis, initiating a rise in haematocrit. Total blood volume and oxygen transportation capacity is increased after weeks of hypoxia-stimulated erythropoiesis. Although physiologically feasible with erythropoiesis-stimulating agents (ESA), there are no current pharmacological strategies to increase haemoglobin concentration recommended by leading HAI guidelines[2].
Figure 1. Formula for tissue oxygen delivery. Circulatory component is in red, haematological component is in purple, and ventilator component is in blue.
CASE DESCRIPTION
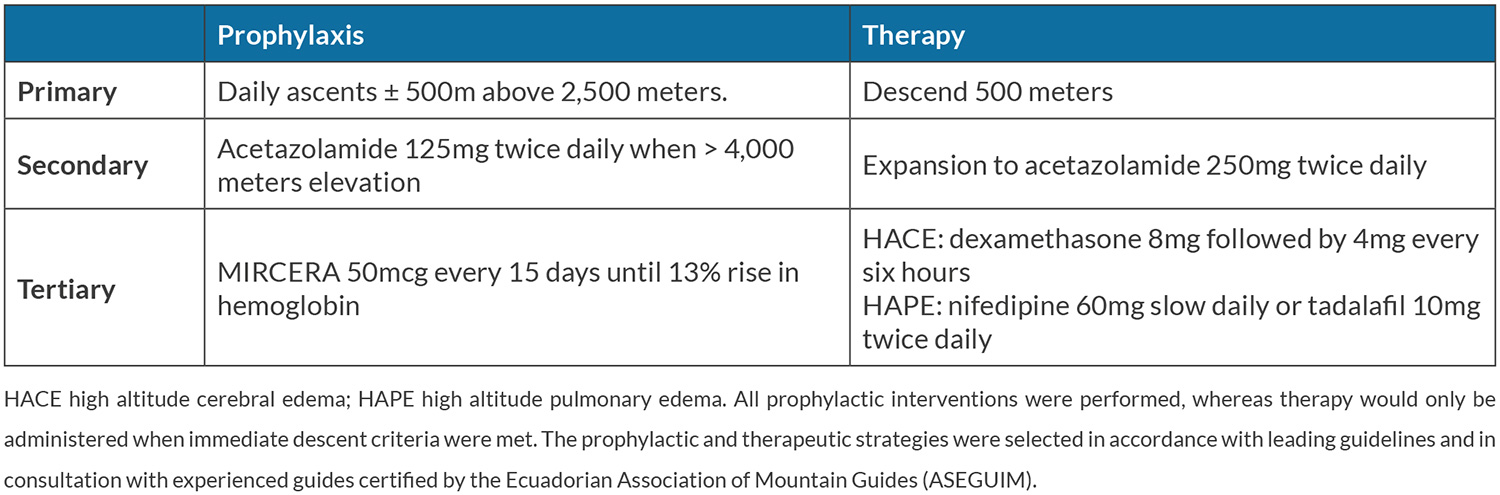
Two 28-year-old healthy and physically fit men planned to climb Mount Chimborazo (6,268 m elevation) in Ecuador over a 12-day period. The first day of ascent consisted of a flight from Curaçao (sea-level) to Quito (2,850 m elevation) followed by three incremental acclimatization ascents prior to the Mount Chimborazo ascent. The subjects are sea-level inhabitants who were unable to pre-acclimatize and did not have access to hypobaric chambers. Both have basic mountaineering experience, but had experienced moderate headache, mild dizziness, and severe fatigue 2 years earlier while climbing a mountain in the Andes region at 4,900 m. The ascent characteristics and known susceptibility put the subjects at increased risk of HAI. Thus, prophylactic and therapeutic pharmacological strategies, including low-dose acetazolamide (125 mg twice daily), were selected in accordance with leading guidelines (Table 1)[2]. An ESA was selected as haematological pre-acclimatization in consultation with experienced nephrologists and internal medicine physicians. Ethical approval for this study was obtained from the local institutional review board. Both subjects provided informed consent.
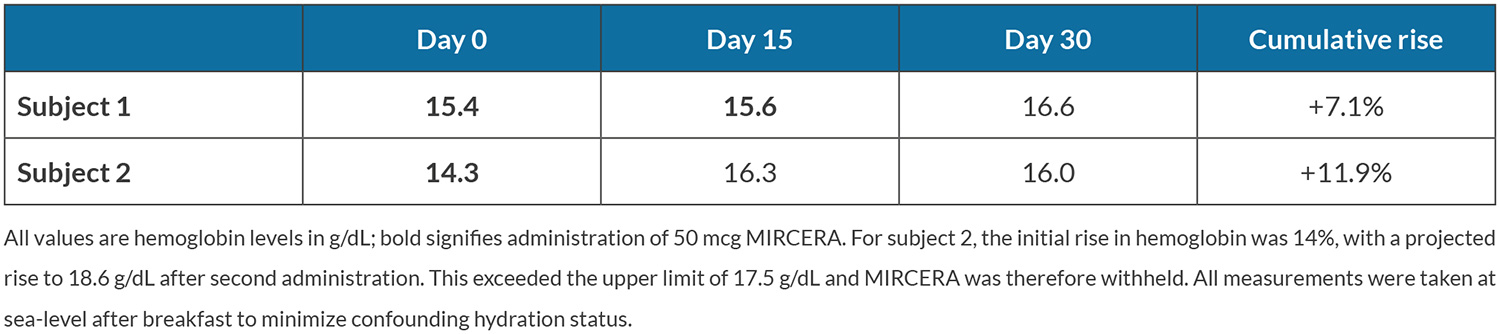
Haemoglobin levels were determined 30 days before arrival in Quito and 50 µg of methoxy polyethylene glycol-epoetin beta (MIRCERA®, Roche, Switzerland), a long-acting ESA, was subcutaneously injected in both subjects. Single dose 75 µg injections of this ESA in healthy male subjects cause a haemoglobin rise and peak of 3–6% after 10 days and immature reticulocyte fraction normalizes after 15 days[3]. Thus, haemoglobin level was measured after 15 days and ESA administration was repeated if the projected rise did not exceed the upper haemoglobin limit (set arbitrarily at 17.5 g/dl).
Parameters of HAI symptomology, the Lake Louise Score (LLS) and immediate descent criteria (LLS>8 or any symptomology suggestive of high altitude cerebral or pulmonary oedema) were evaluated at four basecamps (B) and four summits (S). Peripheral capillary oxygen saturation and heart rate were measured using a pulse oximeter (FPO-10, FYSIC, the Netherlands).
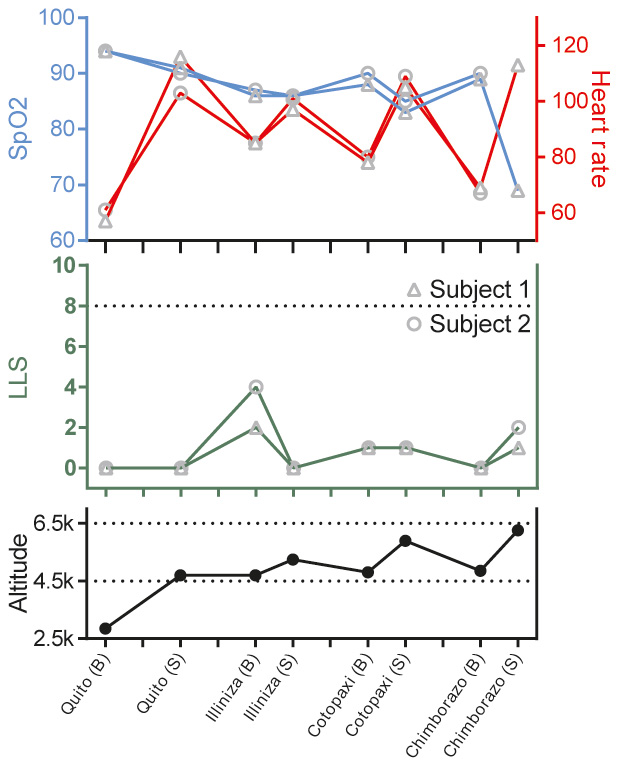
Table 2 shows the time course of haemoglobin values in the 30 days prior to ascent. Fig. 2 shows outcome variables at incremental altitudes during ascent (also see Supplemental Table S1 at https://figshare.com/s/c5b9e4b2d0ae02b4c185). LLS, ranging from 0 to 15, was highest at the Illinizas (5,248 m elevation) being 2 and 4 for subjects 1 and 2, respectively. Experienced symptoms were minor, including headache, dizziness and difficulty sleeping. The immediate descent criteria were never met: LLS remained substantially lower than 8 and no symptoms of cerebral or pulmonary oedema were present. No adverse events occurred.
Figure 2. Outcome variables of subjects at incremental altitudes during ascent. The pulse and SpO2 of subject 2 on the Chimborazo summit could not be measured as the saturation meter's screen froze and was subsequently unusable. Stay at a summit never exceeded 15 minutes and was amidst physical exercise, whereas basecamp stay was 8 hours at a minimum and during rest. The horizontal dotted line in the LLS graph represents an LLS of 8, an immediate descent criterion. Altitude is in meters and heart rate is in beats per minute. B, basecamp; LLS, Lake Louise Score; S, summit; SpO2, peripheral oxygen saturation.
DISCUSSION
HAI causes a large proportion of climbers' deaths and likely contributes to additional deaths currently attributed to injuries, exhaustion and hypothermia. This report describes the combination of haematological pre-acclimatization through an ESA in the preparation stage with (non-)pharmacological strategies during ascent. This approach may diminish the potentially disastrous consequences of HAI and reduce time spent in dangerous acclimatization conditions. Compared with alternative pre-acclimatization strategies, ESA is less costly, less time-consuming, and much more accessible.
Although ventilatory and circulatory ascent strategies have long been accepted as safe and useful, the haematological approach has not. The controversy surrounding ESA stems from its use for 'blood doping' by 1990s professional cyclists and the concurrent war on doping, but empirical evidence for its (long- or short-term) adverse effects in that context is largely unsubstantiated[4]. Nonetheless, use of ESA is not without risk. Increased blood viscosity, blood pressure, coagulation, endothelial activation, platelet reactivity, and inflammation due to ESA use combined with dehydration, hyperthermic exercise, and high-altitude conditions could predispose to thrombotic events[5]. In clinical studies, metabolic, hormonal and renal effects of regularly dosed erythropoietin remained small, reversible, and do not seem to range beyond physiologically acceptable limits[6]. Accordingly, ESA administration in this case report was well-monitored and without adverse effects.
The cumulative haemoglobin rise observed in both subjects (7.1% and 11.9%, respectively) is consistent with one previous study that showed a similar haemoglobin rise as well as lower LLS in response to erythropoietin administration for an ascent to 4,130 m over 7 days[7]. In the current case report, both subjects experienced minimal symptoms during ascent to 6,268 m over 12 days. More research is needed to evaluate the safety and use of ESA as a pre-acclimatization strategy for altitude exposure, and whether its effect is comparable to existing strategies.
Several limitations must be acknowledged. Global oxygen delivery as a singular solution to HAI is likely an oversimplification. Dysfunction of cellular pathways, mitochondria, and (micro-)distribution of oxygen flux may all limit oxygen consumption and contribute to final risk of HAI. Most importantly, this study design lacks both size and controls, and therefore does not allow any robust conclusion on the prophylactic effects of an ESA for HAI. However, it serves as a practical example of implementation based on underlying physiological principles.
CONCLUSION
Ascent to a hypobaric atmosphere without acclimatization can cause HAI with potentially lethal consequences. HAI risk mitigation is based on ventilatory and circulatory modulation. Despite the pathophysiological rationale, there is limited evidence for haematological strategies. This case report presents an ascent strategy including pre-acclimatization using an ESA. In two subjects haemoglobin rose 7.1% and 11.9%, respectively, similar to pre-acclimatization alternatives. No adverse events occurred, and the subjects ascended from sea level to 6,268 m in 12 days with minimal symptoms. Administration of an ESA may be a safe and useful pre-acclimatization strategy but cannot be recommended based on current evidence. Future research should comprehensively investigate whether ESAs can be employed as a pre-acclimatization strategy for mountain climbers.