ABSTRACT
Japanese encephalitis virus is an RNA flavivirus and one of the rare pathogens that can cause encephalitis. The main vector is the Culex tritaeniorhynchus mosquito. The virus is very close in pathophysiology and structure to the West Nile and St. Louis encephalitis viruses. It is endemic in Asia and Western Pacific areas, mostly during the summer; only a few cases have been reported outside those regions. We present the case of a young Filipino cruise line male worker with signs and symptoms of Japanese encephalitis concomitantly with Miller Fisher syndrome and Bickerstaff brainstem encephalitis. The patient developed obtundation, ataxia, areflexia, flaccid paralysis, and ophthalmoplegia, which were preceded by a few days of constitutional symptoms (fever, malaise, fatigue and anorexia). Physical examination showed various stages of erythema nodosum on the lower extremities. Analysis of cerebrospinal fluid was positive for anti-GQ1b, West Nile IgG and Japanese encephalitis IgM. Despite the neurological complications and bradyarrhythmia occurring during hospitalization, the patient recovered completely under our regimen.
LEARNING POINTS
- Insidious onset of bilateral paralysis preceded by fever is most likely encephalitis.
- Japanese encephalitis virus led to the development of variant forms of Guillain-Barré syndrome in our patient.
- Supportive care resulted in significant recovery despite the severity of the condition.
KEYWORDS
Anti-GQ1b, ataxia, Bickerstaff, HIV, Japanese encephalitis, Miller Fisher, West Nile
INTRODUCTION
Japanese encephalitis (JE) virus is an important pathogen causing vaccine-preventable encephalitis in Asia and the Western Pacific area[1]. The virus is a flavivirus, single-stranded RNA virus measuring about 50 nm, and is very similar to the West Nile and St. Louis encephalitis viruses. There are five genotypes of the JE virus, with genotype 1 being the most common[1–3].
The vector is the Culex tritaeniorhynchus mosquito, and the life cycle involves both mosquitoes and vertebrate hosts, particularly swine and wading birds[1–3]. Humans are deemed incidental or dead-end hosts and are usually infected during the summer in subtropical and tropical areas[1–3]. There are approximately 50–170,000 cases of JE worldwide per year, nearly 20–30% of patients die, and 30–50% survive with residual neurological complications[1–3].
The clinical presentation of JE varies, ranging from asymptomatic to severe fulminant encephalitis with very high mortality and morbidity[3]. The incubation period is 6–14 days[3]. Initially, viraemia develops, leading to the progression of constitutional symptoms such as fever, poor appetite, malaise and fatigue[3,4]. Paediatric patients present with abdominal pain, nausea, vomiting, diarrhoea and fever[4]. Some of the possible neurological sequelae include paralysis, areflexia, weakness, and other lower motor neuron symptoms[4]. A few cases diagnosed with JE are associated with Guillain-Barré syndrome (GBS)[4].
Our patient developed Miller Fisher syndrome (MFS) and Bickerstaff brainstem encephalitis (BBE) concomitantly with JE. He experienced ataxia, areflexia, ophthalmoplegia, altered mental status, flaccid paralysis, ptosis, diplopia and impaired sensation. These are the characteristics of MSF and BBE[5]. The patient recovered with supportive measures only.
CASE DESCRIPTION
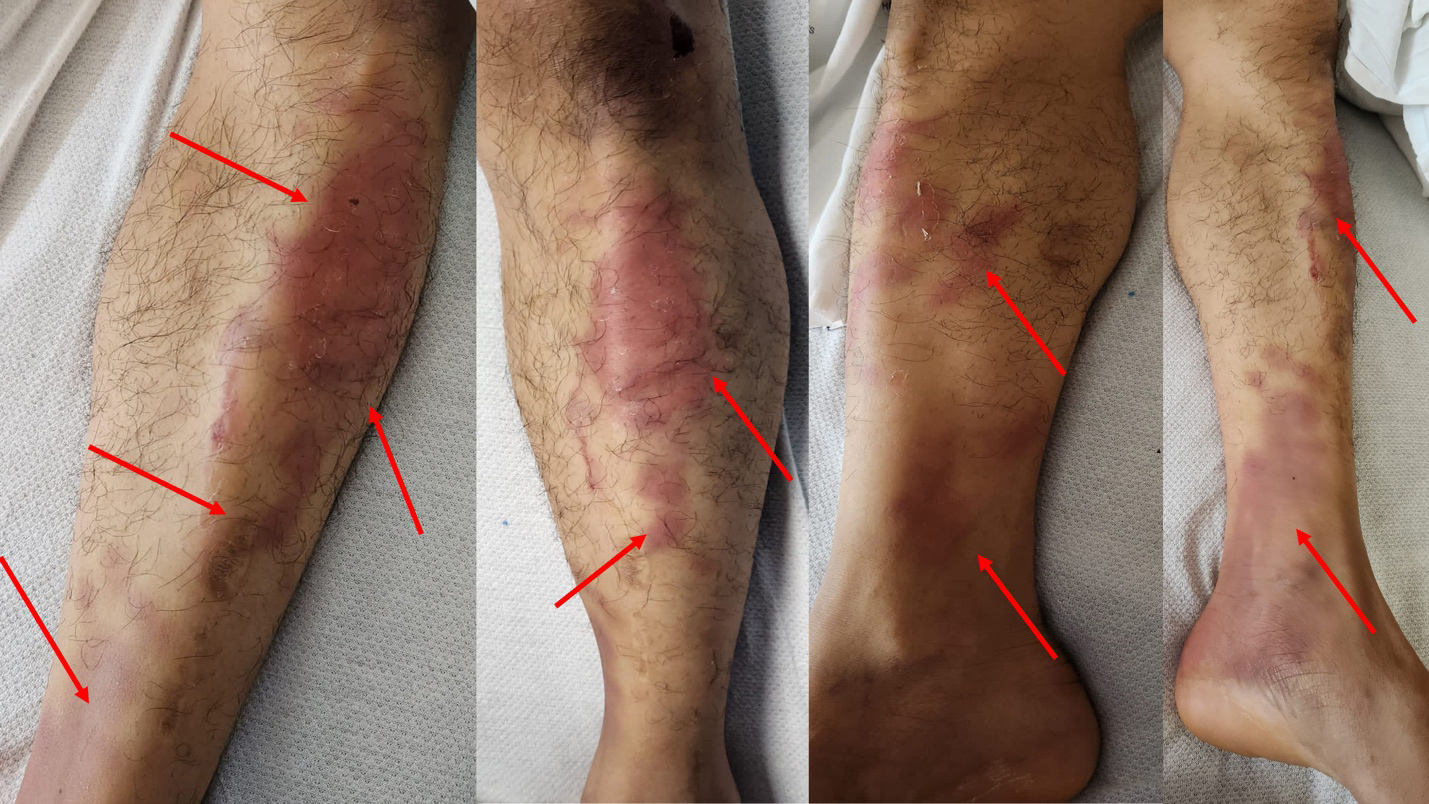
A 27-year-old male Filipino cruise line worker presented to our hospital with altered mental status, diplopia and ataxia, and a 3-day history of fever. Two hours later, he developed obtundation, weakness and agitation. The patient’s vital signs were within normal parameters apart from a low-grade fever of 38.8°C. Physical examination showed rhythmic, slow up-and-down movements of the eyes, increased flaccid paralysis of the lower extremities compared with the upper limbs, and loss of sensation in the upper and lower extremities bilaterally. The Babinski sign was negative and areflexia was noted in all extremities. Brudziński’s and Kernig’s signs were equivocal due to altered mental status. Furthermore, multiple erythema nodosum lesions (Fig. 1) were noticed on physical examination of the lower extremities. We suspected encephalitis (due to viral rather than a less-likely bacterial cause) associated with a variant form of GBS given the lower motor neuron findings.
Figure 1. Multiple erythema nodosum lesions (red arrows) were seen on the lower extremities
The patient was started on intravenous broad-spectrum antibiotics (vancomycin 1g every 12 hours and ceftriaxone 2g every 12 hours) and an antiviral (acyclovir 1g every 8 hours), as well as dexamethasone 20mg. The rapid initial investigation, including peripheral blood work-up, electrocardiography and urinalysis, was unremarkable. A computer tomography (CT) scan of the brain did not show any acute intracranial haemorrhage, lesions or ischaemic changes. The fundoscopic examination was unremarkable. Cerebrospinal fluid (CSF) analysis showed a normal glucose level, high protein (65, normal value 15–45 mg/dl), increased white blood cell count (20, with lymphocytes predominantly, normal value <5), and increased red blood cell count (4000, normal value <10), which was most likely traumatic, and a normal opening pressure. We further investigated the patient for multiple viruses and bacterial infections, as well as CSF-specific proteins for variant GBS.
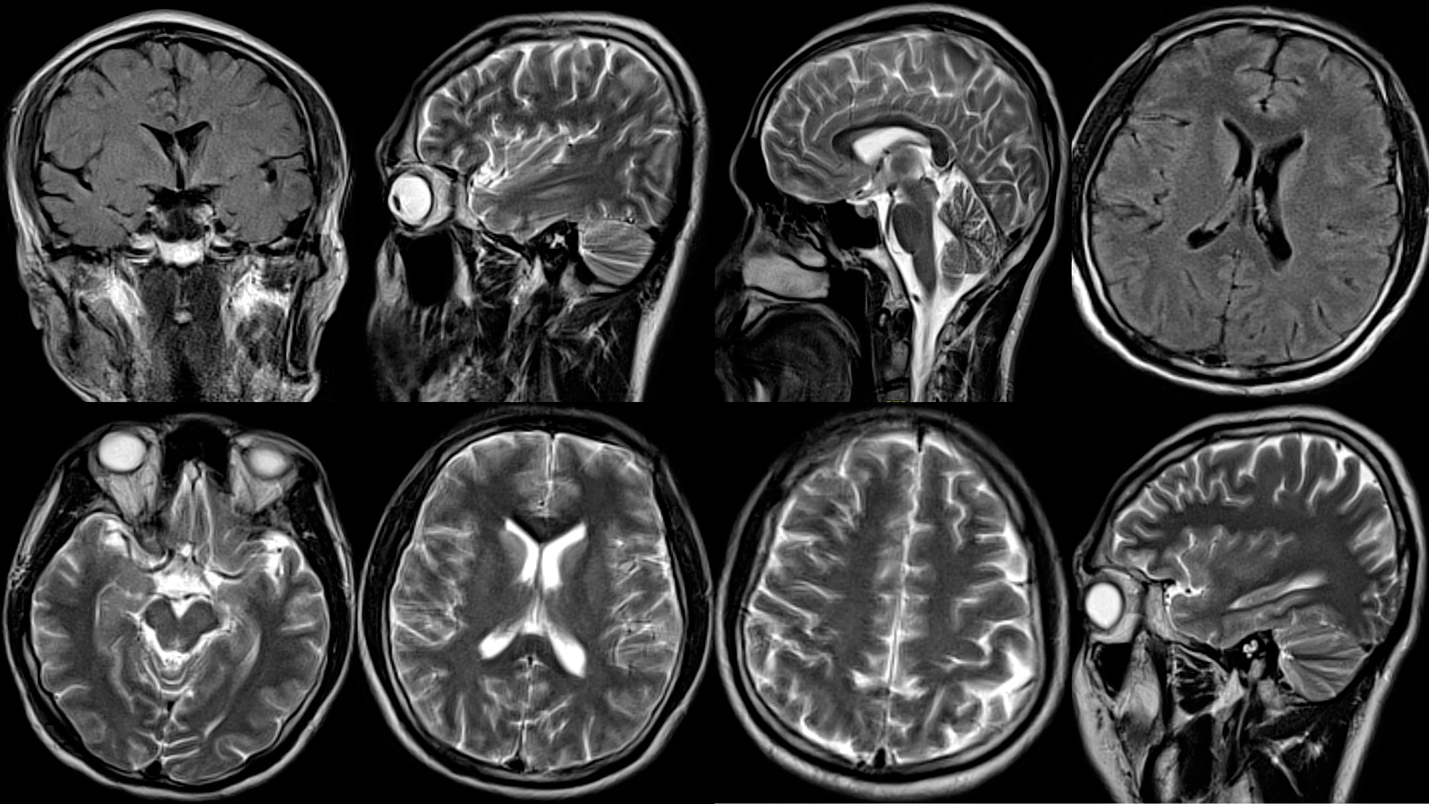
The next day, the patient’s condition remained unchanged with the same findings as previously on physical examination and stable vital signs. The antibiotics and dexamethasone were discontinued given the CSF analysis result, although the patient was kept on acyclovir and supportive care. Brain MRI was unremarkable (Fig. 2). On the third day, the patient developed bradycardia (45–50 beats per minute) and mild transaminitis (aspartate aminotransferase (AST) 106, normal level 10–45 U/l, and alanine transaminase (ALT) 67, normal level 8–40 U/l). We decided to observe the patient closely. Vital signs (apart from the pulse rate) were within normal limits. It was noteworthy that the viral screen for herpes simplex 1 and 2, cytomegalovirus, Epstein–Barr virus, Zika, dengue, human immunodeficiency virus, yellow fever virus, St. Louis encephalitis virus, varicella zoster and mumps came back negative. However, positive results were obtained for West Nile virus (IgG only) and for JE virus (IgM). All bacterial investigations came back negative, including Legionella, Mycoplasma, Chlamydia, Treponema pallidum, Streptococcus and Staphylococcus aureus. On the fourth day, the erythema nodosum slightly improved, bradycardia resolved, areflexia became hyporeflexia, sensation improved, the tone of the extremities slightly improved, and the patient was able to answer simple questions and follow commands. Additionally, asialo antibodies (GQ1b, ganglioside-monosialic acid antibodies) were detected at 250 IV (normal level 0–50). On the fifth day, the patient’s condition improved significantly with supportive care only, and we added plasmapheresis to the regimen. On the sixth day, the flaccid paralysis, sensation impairment and hyporeflexia resolved.
DISCUSSION
The development of GBS after infection with different pathogens is not well understood[4,5]. The pathophysiology involves molecular mimicry (viral particles), the myelin sheath, and inflammatory cells (macrophages and lymphocytes) which attack the myelin sheath and neurons in the peripheral nervous system, leading to demyelination of the axons[4,5].
MFS is characterized by the triad of ataxia, areflexia and ophthalmoplegia[4–6], while BBE is distinguished by the triad of ataxia, ophthalmoplegia and altered sensorium[4–6]. Both conditions are associated with anti-GQ1b antibodies[4–6]. The pathophysiology of the variants of GBS (MFS and BBE) is not well understood[4–6]. They can be induced by different triggers/pathogens, including idiopathic, viral and bacterial causes, drugs (heroin, suramin, streptokinase, isotretinoin, tumour necrosis factor alpha inhibitors), autoimmune diseases (lupus, sarcoidosis), surgery, bone marrow transplantation, and even vaccination[6–8]. Rarely, MFS has occurred after COVID-19, Campylobacter jejuni, Zika virus, herpesvirus 8, Norovirus, human immunodeficiency virus, and Epstein–Barr virus infection[6,7,9,10].
JE virus infection consists of four phases: (a) the prodromal phase (constitutional symptoms and gastrointestinal symptoms); (b) acute altered mental status, seizures (mostly in children) and neurological/behavioural symptoms; (c) temporary extrapyramidal symptoms such as tremor, rigidity, dystonia, and other movement disorders, which occur in less than 50% of cases; and (d) neurological sequelae[5,7,8]. Further, about 15% of patients developed opisthotonic posturing, and 20% of infected individuals exhibited weakness, flaccid paralysis, and areflexia when the anterior horn neurons of the spinal cord were affected[5,7,8]. The mortality rate is about 20–30%, and most patients die during the acute phase due to increased intracranial pressure or neurogenic pulmonary oedema[5,7,8]. Half of patients with a positive anti-GQ1b finding developed bradyarrhythmias due to parasympathetic overactivity[10]. This could be related to unregulated vagal nerve firing versus failure of the baroreceptor reflex of the afferent limb[10].
The diagnosis can be established via cerebrospinal fluid analysis (JE IgM ELISA) and electroencephalography, which can show theta and delta waves, alpha coma, and burst suppression patterns during the acute phase[5,7,8]. Brain MRI may show normal brain versus T2 and fluid-attenuated inversion recovery (FLAIR) hyperintensities in the basal ganglia, substantia nigra, pons, thalami and hippocampus[5,7,8]. Further, acute disseminated encephalomyelitis (ADAM) can also be observed in acute severe JE[3,7].
Currently, no treatment is available for JE[1,3,7] and infected individuals are treated with supportive care only[1,3,7]. However, vaccination can prevent the disease[1,3,7]. Four types of vaccines are available: inactivated mouse brain-derived vaccine, inactivated Vero cell vaccine, live attenuated vaccine, and chimeric vaccine[1,3,7]. For variant forms of GBS (MFS and BBE), immunoglobulin or plasmapheresis along with supportive care is recommended[1,3,7].
CONCLUSION
Given the potentially catastrophic implications of this infection, further research on the JE virus as well as on the pathophysiology of its concomitant occurrence with GBS derivatives (MFS and BBE) is warranted.