ABSTRACT
Introduction: While T-wave inversions (TWI) are associated with various pathologies, they are rarely associated with cardiac memory, termed the Chatterjee phenomenon.
Case: A 76-year-old man with sick sinus syndrome with a pacemaker presented with chest tightness and new onset TWI in his precordial leads. On admission, he tested positive for COVID-19, but remained stable and only required minimal supplemental oxygen. His troponin was only slightly elevated, and EKG showed TWI throughout his precordial leads. A previous EKG had shown normal sinus rhythm without a paced rhythm or ST wave abnormalities. Interrogation of his pacemaker revealed an AV-paced rhythm. Given his chest tightness without dynamic changes in his troponin or EKG, the symptoms were considered more likely related to his COVID-19 infection, and he was discharged home.
Discussion: Aberrancies in normal cardiac conduction can result in altered electrical activation, especially for those with AV pacemakers, leading some patients to develop cardiac memory, manifesting as TWI.
Conclusion: AV-paced rhythm and narrow QRS complexes with TWI localized to precordial leads without evidence of active cardiac ischaemia may suggest cardiac memory, termed the Chatterjee phenomenon, requiring no invasive interventions.
LEARNING POINTS
- In patients with T-wave inversions, various conditions should considered in the differential diagnosis, including left bundle branch block and sick sinus syndrome, although T-wave inversions in V1–V3 are non-specific and benign.
- Cardiac memory, termed the Chatterjee Phenomenon, is one of the causes of T-wave inversions which is sometimes ignored.
- No invasive interventions are needed for T-wave inversions with the Chatterjee phenomenon.
KEYWORDS
Cardiac memory, T-wave inversion, pacemaker
INTRODUCTION
It is known that right ventricular pacing can induce both electrophysical and mechanical changes in the myocardial tissues that result in cardiac memory, known as the Chatterjee phenomenon. This ventricular cardiac memory manifests as T-wave inversions (TWI) due to a reflection of the previous QRS complex[1]. While TWI in V1–V3 are non-specific and benign[2], they are also commonly associated with hypokalaemia, ischaemia, and hypertrophic obstructive cardiomyopathy; however, cardiac memory TWI are due to altered electrical activation[3]. We report the case of a 76-year-old man with known sick sinus syndrome (SSS) and a DDD pacemaker with a recent COVID-19 infection who presented to the hospital with new-onset TWI.
CASE DESCRIPTION
A 76-year-old man with SSS with a DDD pacemaker and heart failure with preserved ejection fraction (HFpEF) presented with chest tightness and pain lasting about an hour. The patient reported that the pain extended across the epigastric and right hypochondriac regions and worsened with respiration but not upon exertion. Seven days before admission, the patient had received his second COVID-19 vaccination. Four days before admission, he first experienced chest tightness, a non-productive cough, and shortness of breath but remained afebrile. He tested positive for COVID-19 upon admission with mild hypoxia. Chart review revealed that his echocardiogram from 2 months previously showed mild diastolic dysfunction with an ejection fraction of 55–60%, suggestive of HFpEF. In the emergency department, the patient presented with hypertensive urgency with a blood pressure of 200/98 mmHg, which resulted in mildly elevated troponins, likely due to demand ischaemia without further elevation. Chest x-ray showed mild cardiomegaly with vague right perihilar, and basilar infiltrates.
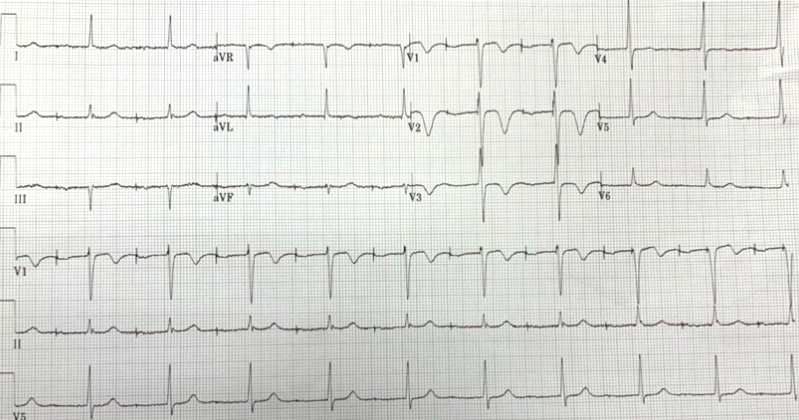
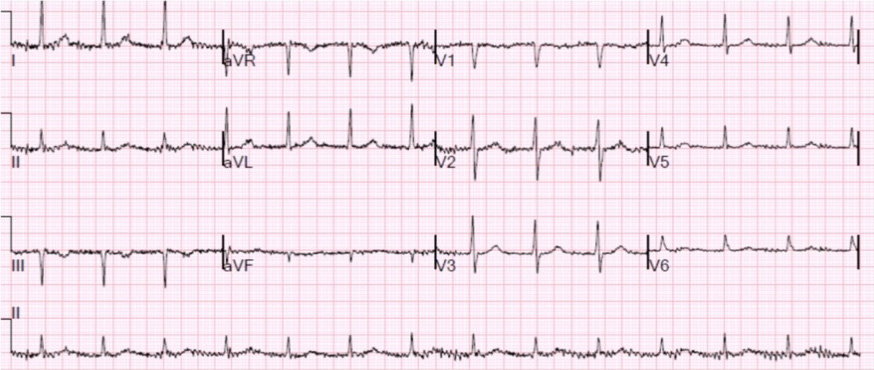
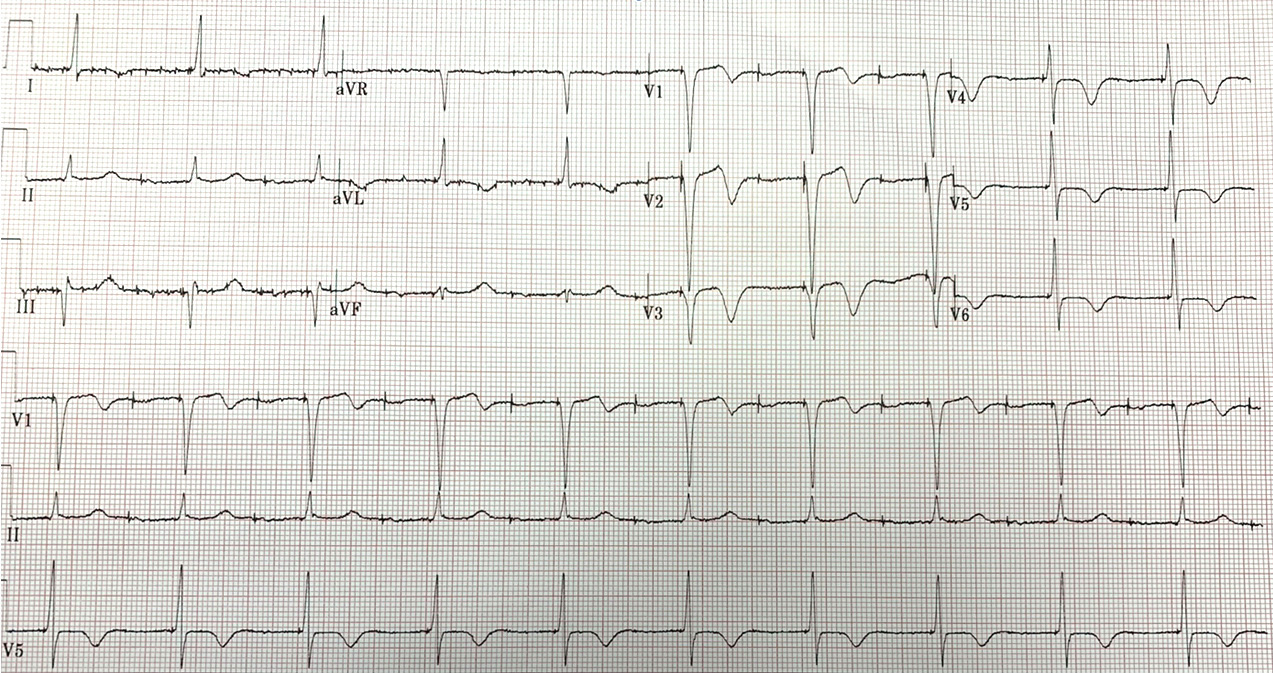
Electrocardiogram (EKG) revealed an atrioventricular (AV)-paced rhythm and anteroseptal TWI in V1–V3 which could be non-specific with no ST changes (Fig. 1). Pacemaker interrogation disclosed atrial and ventricular paced rhythm at 60% and 45%, respectively, with no pacemaker-mediated tachycardia. His previous EKG from 1 year earlier did not demonstrate the TWI mentioned above (Fig. 2). He was subsequently admitted for observation for COVID-19 pneumonia. Repeat EKG showed TWI in V1–V6 (Fig. 3), which was similar to the initial EKG and suspected to be due to the automaticity of the heart in the setting of a pacemaker. A cardiology consult assessed the patient and deemed the chest tightness not to be cardiac and likely related to his COVID-19 infection. The following day, his chest pain resolved and the patient was discharged after a 1-day observation.
Figure 2. Previous electrocardiogram without notable T-wave inversions in anteroseptal leads
DISCUSSION
Aberrancies in normal cardiac conduction can result in altered electrical activation, especially for those with AV pacemakers, leading some patients to develop cardiac memory, manifesting as TWI. Chatterjee et al. noted that TWI and ST depressions for patients undergoing ventricular pacing correlated with the length and electrical power of the ventricular pacemaker, and TWI were attributed to the actual ventricular depolarization rather than any intrinsic effects of the pacemaker[4].
After further characterization, TWI have been noted in other ventricular abnormalities, such as left bundle branch block, Wolff-Parkinson-White syndrome, and SSS[1,5,6]. A previous study also suggested that the electrical modulating effects of ventricular repolarization, rather than depolarization, were the underlying cause of TWI, termed cardiac memory[7]. The term cardiac memory reflects the underlying principle that the direction of the T-wave should reflect the overall previous abnormal deflection of the QRS complex[8]. While the exact mechanism of cardiac memory is yet to be elucidated, molecular and electrophysiological studies on animal subjects suggested that altered activation might influence both early and late myocardial activation potentials[9]. In terms of early activation potentials proximal to the activation site, blockade of angiotensin II receptors could attenuate the proximal epicardial phase 1 notch action potential via the downregulating expression of cardiac ion channels such as delayed rectifiers and connexin 437. The altered expression of these ion channels occurring in the proximal site, rather than the distal site of myocyte activation, has been attributed to cardiac memory and the observed TWI[3]. In terms of late-activated action potentials for distal myocyte cells, cardiac memory was speculated to be due to increased mechanical strain. Combining early and late localization of myocardial action potentials allows for cardiac remodelling and heterogenous TWI.
With regards to our patient, given the patient’s medical history and previous wide QRS complexed ventricular-based rhythms, it is more plausible that his new TWI can be attributed to cardiac memory, which requires no invasive interventions.