ABSTRACT
Sickle cell disease is a prevalent hematologic condition, but some of the factors that lead to erythrocyte sickling are not fully known. A 58-year-old male patient with a history of sickle cell disease (SCD) and paroxysmal atrial fibrillation was transferred from an outside hospital for further management of refractory sickle cell crisis with acute chest syndrome. Before transfer, the patient received antibiotics and multiple packed red blood cell (pRBC) transfusions, with minimal effect on symptoms or anemia. After transfer, the patient developed rapid supraventricular tachycardia and atrial fibrillation (rates >160) with a drop in blood pressure. He was started on IV amiodarone. His heart rate was subsequently better controlled and converted to sinus rhythm the following day. Three days following initiation of amiodarone, the patient, with a hemoglobin count of 6.4 g/dl, required one additional unit of pRBC. On the fourth day, the patient’s hemoglobin count rose to 9.4 g/dl, and he reported a marked improvement in symptoms. The improvements in symptoms and hemoglobin count were sustained, and the patient was discharged two days later. This remarkable improvement in anemia and symptoms triggered a search for potential causes. Amiodarone is a complex drug shown to have effects on multiple cell types, including erythrocytes. A recent preclinical study demonstrated reduced sickling and improved anemia in a murine model of SCD. This case report raises the possibility that amiodarone may have contributed to the rapid improvement in anemia and should be further explored in clinical trials.
LEARNING POINTS
- Prior studies support a link between erythrocyte sickling and membrane lipid composition.
- Amiodarone may impact erythrocyte pathophysiology by increasing cellular lipids including bis(mono)acylglycerol phosphate (BMP).
- Drugs with effects on erythrocyte lipid fractions may be beneficial during sickle cell crises.
KEYWORDS
Amiodarone, sickle cell disease, anemia, erythrocytes
INTRODUCTION
Sickle cell disease is a relatively common inherited blood disorder in the United States and around the world. The condition arises from a missense mutation of the β-globin gene, resulting in the substitution of valine for glutamic acid at position 6 of the β-globin chain[1]. The mutated hemoglobin tetramer polymerizes following deoxygenation, forming a network of fibrous polymers that causes sickling in erythrocytes[1]. These sickled red blood cells are more easily destroyed, which leads to hemolytic anemia[1]. Sickle cell pain crises arise due to microvascular occlusions[2]. In addition, episodic vaso-occlusions may lead to acute coronary syndrome, stroke, pulmonary hypertension, and renal failure[2].
CASE DESCRIPTION
A 58-year-old male with a history of sickle cell disease (SCD), avascular necrosis of the humerus and femur, pulmonary hypertension with right heart failure, chronic kidney disease, paroxysmal atrial fibrillation, and deep vein thrombosis/pulmonary embolism presented to an outside hospital with symptoms of chest pain and shortness of breath and was diagnosed with a sickle cell pain crisis.
Chest X-ray demonstrated prominence of the pulmonary interstitium, likely due to congestion/mild edema. Electrocardiogram revealed sinus tachycardia with no ST segment changes. He was started on doxycycline, vancomycin, and cefepime and admitted for further management. Hemoglobin (Hgb) down trended to 5.8 g/dl, and he was transfused with three units of pRBCs. His pain remained uncontrolled, requiring morphine via patient-controlled analgesia. He subsequently became diaphoretic, tachycardic, and tachypneic. His respiratory status declined, and his oxygen requirements increased to 15 l. He was intubated and transferred to a university hospital intensive care unit, where hematologists diagnosed acute chest syndrome, as he developed fever and chest pain. A chest X-ray showed bilateral patchy consolidations.
Four days later, the patient developed episodes of supraventricular tachycardia and atrial fibrillation with rapid ventricular responses (rates > 160) accompanied by hypotension. The patient was administered intravenous amiodarone with continued intravenous heparin. After intravenous administration of 150 mg of amiodarone, a drip was started at 1 mg/minute. He was later transitioned to oral dosing at 400 mg twice a day to achieve a total 10-gram load followed by maintenance dosing at 200 mg per day.
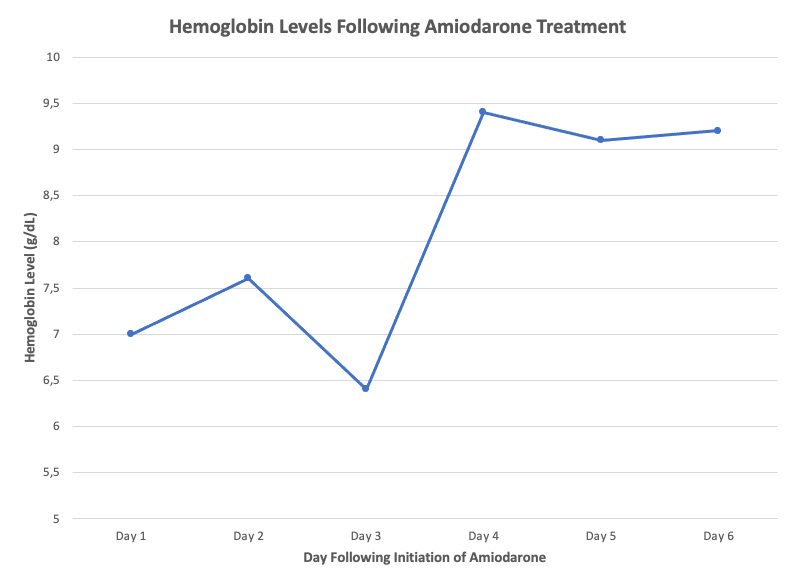
Four days following the initiation of amiodarone, the patient’s hemoglobin count increased from 6.4 g/dl to 9.4 g/dl, and his hematocrit level increased from 19.3% to 28.5%, even though he only received one unit of additional blood during that time period (Fig. 1 and Fig. 2). This improvement in anemia was associated with marked improvement in symptoms, and the patient was discharged two days later.
Following discharge, the patient continued to do clinically well and was maintained on amiodarone therapy for over three months. After a follow-up appointment with an electrophysiologist, the decision was made to discontinue amiodarone due to concerns over potential long-term side effects. Plans were made for an ablation procedure.
Figure 1. Hemoglobin levels following the initiation of amiodarone, up to the time of discharge
Figure 1. Hematocrit following the initiation of amiodarone, up to the time of discharge
DISCUSSION
Multiple factors impact erythrocyte sickling. However, some of the physical characteristics of erythrocytes that predispose patients to sickling remain unclear. The lipid composition of the erythrocyte membranes is critical for the fluid structure and proper function of the cell and may play a role in erythrocyte shape change, including sickling[3]. Amiodarone is an anti-arrhythmic medication that affects multiple cell types, including erythrocytes[3]. Amiodarone has been shown to alter the cholesterol-to-phospholipid ratio in erythrocytes[3].
Four days following the administration of amiodarone, the patient had a rapid improvement in anemia and symptoms related to sickle cell crisis. While the improvement could be related to multiple factors, the rapid time course of recovery raises the possibility that amiodarone contributed to improvement in the patient’s anemia. Amiodarone has been shown to affect erythrocyte membrane lipid composition[3] and to increase bis(mono)acylglycerol phosphate[4]. A preclinical study demonstrated an unexpected worsening of anemia in SCD mice with concomitant deficiency of PCSK9[5]. Erythrocytes isolated from these mice were also more prone to sickling in an ex vivo assay[5]. A shotgun lipidomics analysis of erythrocytes isolated from these mice revealed reduced bis(mono)acylglycerol phosphate (BMP), also known as lysobisphosphatidic acid[6]. BMP is a regulatory lipid that plays a key role in endosomal integrity and function[4]. Although BMP may be altered in various disease states, the causal relationship between BMP and disease pathophysiology remains unclear. However, the study found decreased BMP associated with more severe anemia in erythrocytes from sickle cell mice deficient in PCSK9[6]. Amiodarone increases BMP[4], and treatment with amiodarone improved anemia in SCD mice[6]. This effect was also associated with reduced ex vivo erythrocyte sickling[6].
CONCLUSION
While the side effect profile of amiodarone may preclude long term treatment, this case study suggests that short term amiodarone treatment may represent a useful therapeutic intervention in patients with sickle cell disease during crises. According to the Naranjo Method, it is "probable” that amiodarone led to the improvements in anemia observed[7]. Further clinical studies are warranted.