ABSTRACT
Dasatinib is a tyrosine kinase inhibitor used for treatment of some specific types of leukaemia. The development of pleural effusion is a known adverse effect of dasatinib and chylothorax is exceptional. No case has been reported beyond 5 years of treatment and extensive search for an alternative diagnosis is currently suggested in such scenario. The underlying mechanism is not currently clear. We describe a woman on dasatinib treatment for more than 10 years who developed chylothorax. Drug withdrawal resolved the chylous pleural effusion. We were able to find 14 additional cases of dasatinib-related chylothorax reported up until now.
LEARNING POINTS
- Dasatinib is a tyrosine kinase inhibitor used for the treatment of some specific types of leukaemia.
- Development of pleural effusion is a potential adverse effect, mostly in the first 6 years of treatment. The underlying mechanism is not known.
- Chylothorax is exceptional, and no case had been described beyond 5 years of treatment; our case would be the first one. We found 14 additional cases of dasatinib-related chylothoraces in a PubMed research.
KEYWORDS
Dasatinib, chylothorax, pleural effusion
INTRODUCTION
Dasatinib is a tyrosine kinase inhibitor used for the treatment of oncogene fusion protein BCR-ABL1-positive chronic myeloid leukaemia and Philadelphia chromosome-positive acute lymphoblastic leukaemia[1]. The development of pleural effusion is a known adverse effect of dasatinib, and chylothorax is exceptional[2]. Beyond six years of treatment with dasatinib only 0.8% of patients develop pleural effusion, and no case of chylothorax has yet been described after five years of its use according to a recent review[3]. The underlying mechanism is not currently clear[4]. We describe a woman on dasatinib treatment for more than 10 years who developed chylothorax. Drug withdrawal resolved the pleural chylous effusion. We were able to find 14 cases of dasatinib-related chylothorax reported up until now (ours not included)[3,5]. The patient gave her signed informed consent for the publication of her clinical case.
CASE DESCRIPTION
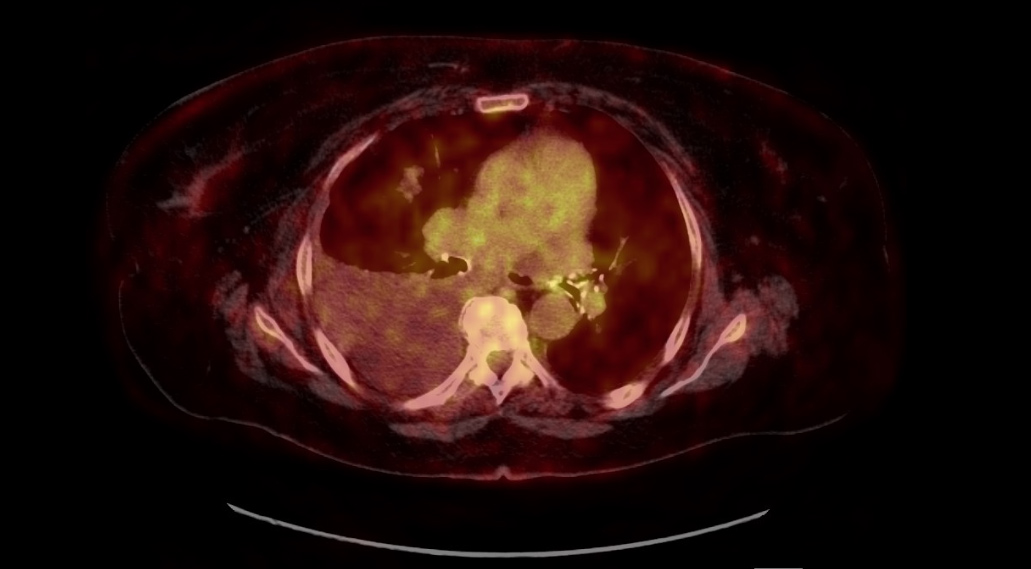
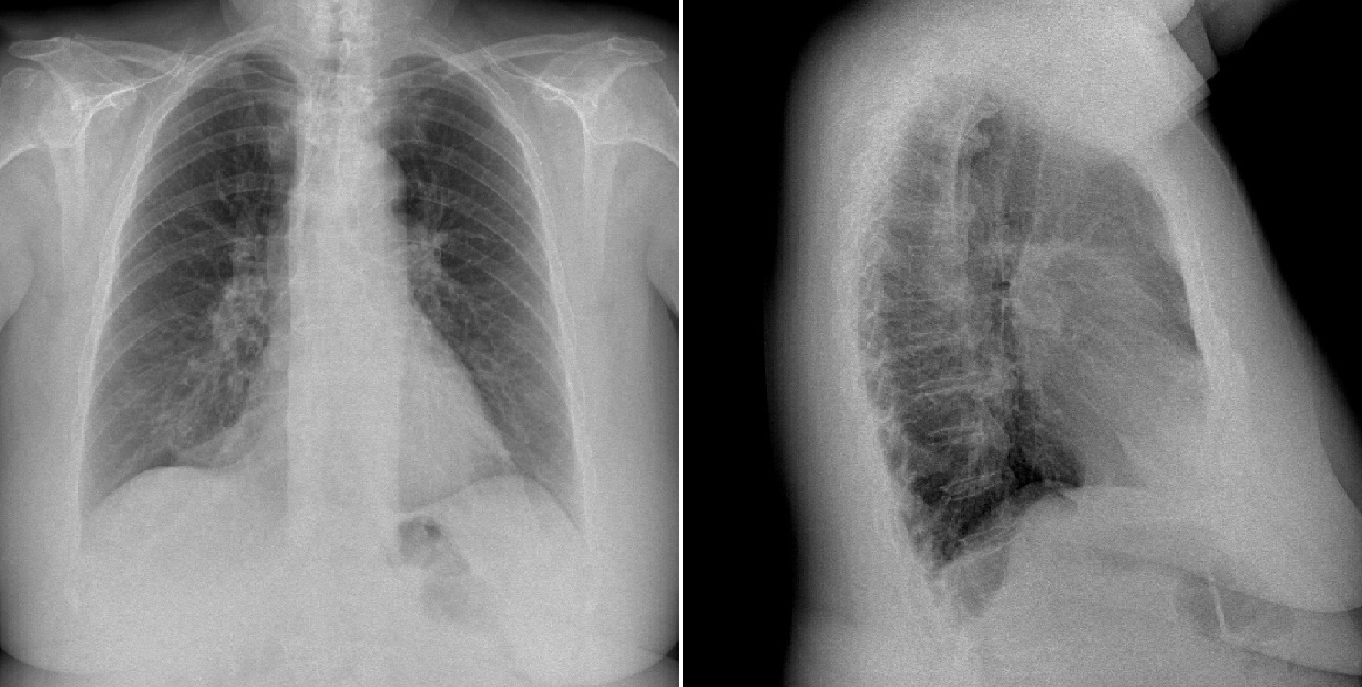
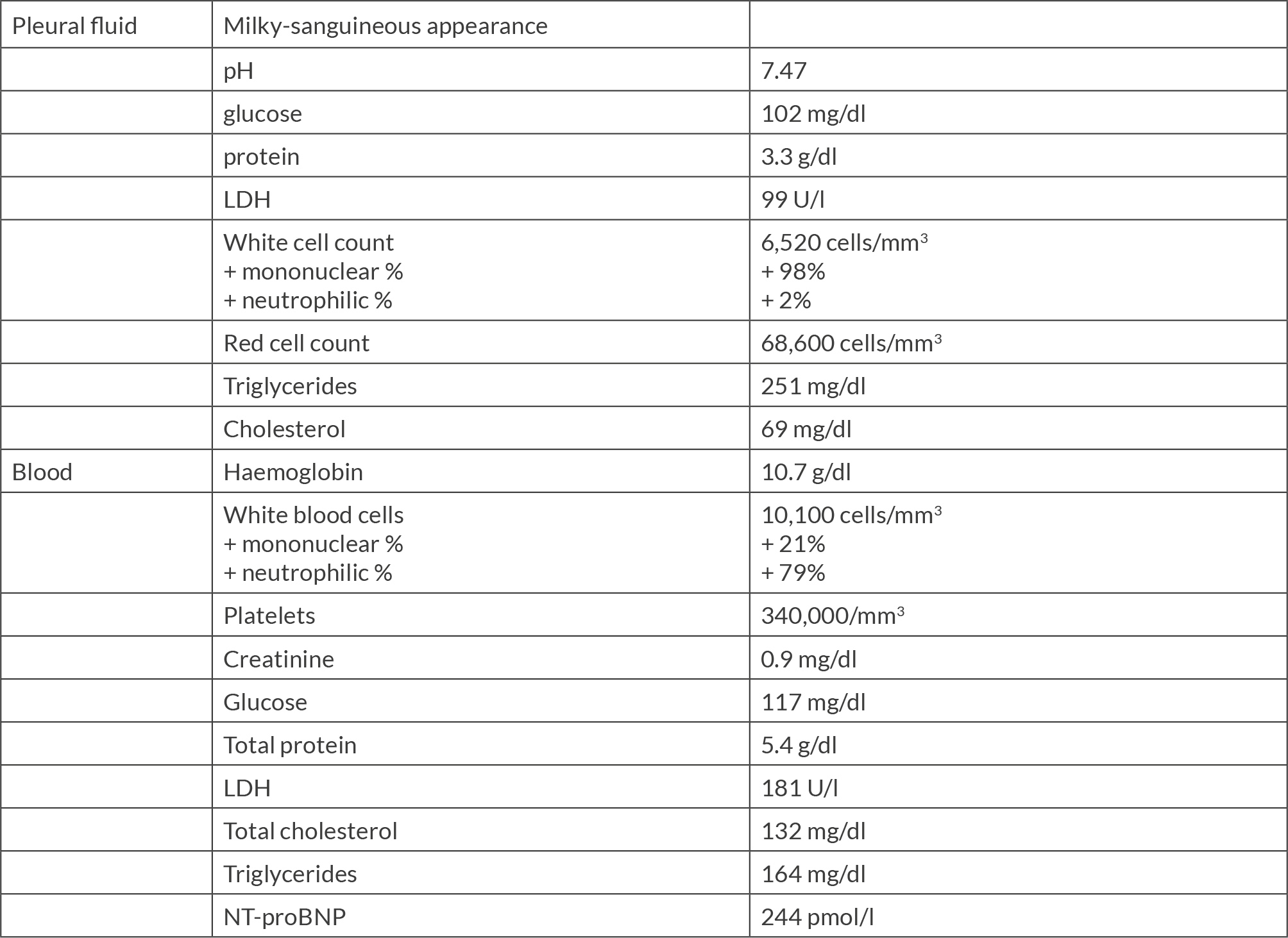
A 72-year-old woman was admitted to our hospital in September 2022 because of dyspnoea for 3 months. She had been diagnosed with chronic myeloid leukaemia in December 2007. Imatinib as the first treatment and nilotinib later were discontinued due to adverse effects and intolerance. From November 2011 she was on dasatinib treatment, with a daily dose of 100 mg. She was also on anticoagulant treatment due to recurrent deep vein thrombosis since 2018. A chest X-ray revealed a right-sided pleural effusion. On physical examination her vital signs were temperature 35.7 ºC, blood pressure 110/50, heart rate 60 beats per minute, respiratory rate 20 breaths per minute and oxygen saturation 96% on room air. On auscultation, a decreased breath sound became evident in the right lung base. The rest of the physical examination was unremarkable. The most relevant laboratory results on admission regarding serum and pleural fluid analysis obtained by diagnostic thoracentesis are shown in Table 1. Pleural fluid values were consistent with a lymphocytic and exudative effusion, fulfilling chylothorax criteria. Thoracic computed and positron emission tomography showed a marked hypomethabolic right pleural effusion without any other remarkable finding (Fig. 1). A thoracic nuclear magnetic resonance did not find any abnormality in the thoracic duct. Transthoracic echocardiography showed a normal size of both ventricles, absence of any abnormality regarding valves and no pericardial effusion. Pleural fluid culture and Xpert MTB/RIF for Mycobacterium tuberculosis were negative, along with cytology and flow cytometry for malignant, monoclonal or lymphoproliferative disease. A Lowenstein-Jensen culture was also negative 8 weeks later. Chylothorax remained stable through the performance of all these procedures, so we decided to discontinue dasatinib treatment and opted for outpatient control. One month after hospital discharge the chest X-ray was normal (Fig. 2).
Figure 1. Positron Emission Tomography only showed an hypomethabolic right pleural effusion
DISCUSSION
Pleural effusion is a relatively frequent complication during dasatinib treatment, with a reported incidence between 14% and 32%[4]. Very infrequently, the pleural effusion developed is a chylothorax.
The mechanism by which pleural effusion develops remains unknown, but inhibition of platelet-derived growth factor B receptor (PDGFR-B) by dasatinib with subsequent decreased interstitial fluid pressure and increased vascular permeability is being seriously considered[6,7]. In this sense, PFGFR-B is known to regulate the angiogenesis, lymphangiogenesis, mesangial and vascular smooth-cell proliferation, along with pericyte recruitment of capillaries, so its inhibition by dasatinib might impair vascular remodelling[8].
The prescription of a single dose of dasatinib per day is associated with a significantly lower risk of pleural effusion development, hypothetically due to a lower mean minimum concentration of the drug in the blood[9]. Other potential factors associated with the development of pleural effusion have been evaluated and reported: patients’ age, lymphocyte count, increased blood pressure, previous cardiac history, previous autoimmune disease, a skin rash due to dasatinib and hypercholesterolemia[10,11].
The mean period for developing pleural effusion was calculated at 60 weeks on dasatinib treatment, and only 0.8% of patients developed pleural effusion beyond 6 years on it[12]. On the other hand, in all reported cases of dasatinib-related chylothorax, patients had been treated for less than 5 years, and most of them developed it in the first 12 months following drug initiation. Therefore, an extensive and broad work-up seemed advisable when chylothorax is diagnosed beyond a period of 5 years of treatment[3].
Management of dasatinib-induced pleural effusions includes stopping the treatment, reducing the dose of the drug, administering diuretics or prescribing a short course of corticosteroids, along with therapeutic thoracentesis and a low-fat diet. Currently there are no specific guidelines in this regard, but there is some evidence pointing out that low and moderate effusions tend to respond well to discontinuation of treatment; high degree effusions usually need all the above-mentioned measures, and replacing dasatinib with imatinib is a reasonable alternative when feasible[3,9].
CONCLUSION
It seems unlikely that there is a time limit for the development of chylothorax in patients on dasatinib treatment. We must pay special attention to any pleural effusion developing in patients treated with such therapies and report them all: recently, even one additional case of chylothorax due to pazopanib treatment for renal cell carcinoma has been reported[13]. Understanding the pathophysiology of this process and subsequently establishing clinical guidelines for its management is a medical challenge which we should all get involved in.