ABSTRACT
Introduction: Hereditary haemorrhagic telangiectasia (HHT) is a rare multi-organ vascular disease. It is characterised by mucocutaneous telangiectasia, epistaxis, and arteriovenous malformations. Some 70% of patients with HHT are thought to have issues with gastrointestinal (GI) bleeding. Traditional management of GI bleeding in HHT includes monitoring for iron deficiency anaemia, iron replacement, antifibrinolytic therapy and control of identifiable bleeding sites with argon photocoagulation during gastrointestinal endoscopy. Blood transfusion may also be required.
Case description: Our case describes a man in his 40s with confirmed HHT, with transfusion-dependent anaemia secondary to GI bleeding. He was commenced on fortnightly bevacizumab (5 mg/kg) for 12 weeks in an attempt to reduce his blood transfusion requirement and manage his anaemia. In the months prior to starting bevacizumab, our patient’s transfusion requirement ranged from 3–5 units of packed red cells per month to maintain an Hb >8 g/dl. He had a marked improvement in his symptoms within the first month of treatment and did not require any further blood transfusion during the three months of treatment. He was given one further IV iron infusion in the final month of his 3-month bevacizumab treatment and did not experience any adverse side effects from bevacizumab.
Discussion: HHT results from alterations to genes which encode proteins involved in blood vessel formation. This provides the rationale for using anti VEGF drugs such as bevacizumab. Current evidence for this treatment approach is limited.
Conclusion: Bevacizumab can be an effective treatment option in patients with HHT refractory to traditional management.
LEARNING POINTS
- Gastrointestinal bleeding in hereditary haemorrhagic telangiectasia can be difficult to treat.
- Bevacizumab, an anti-vascular endothelial growth factor, can be used to treat refractory anaemia secondary to gastrointestinal bleeding in hereditary haemorrhagic telangiectasia.
KEYWORDS
Bleeding, anaemia, bevacizumab, hereditary haemorrhagic telangiectasia, HHT
INTRODUCTION
Hereditary haemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome is a rare multi-organ vascular disease, thought to affect 1 in 5,000–8,000 people worldwide[1]. It is characterised by mucocutaneous telangiectasia, epistaxis, and arteriovenous malformations, which can affect multiple organs. Some 70% of patients with HHT are thought to have issues with gastrointestinal (GI) bleeding[2]. This can result in anaemia, as observed in our case.
Traditional management of gastrointestinal bleeding in HHT includes monitoring for iron deficiency anaemia, oral or intravenous iron replacement, antifibrinolytic therapy with tranexamic acid and control of identifiable bleeding sites with argon photocoagulation at GI endoscopy or cautery with ENT specialists[3]. Blood transfusion is also used as needed. GI bleeding in HHT can be difficult to treat.
CASE DESCRIPTION
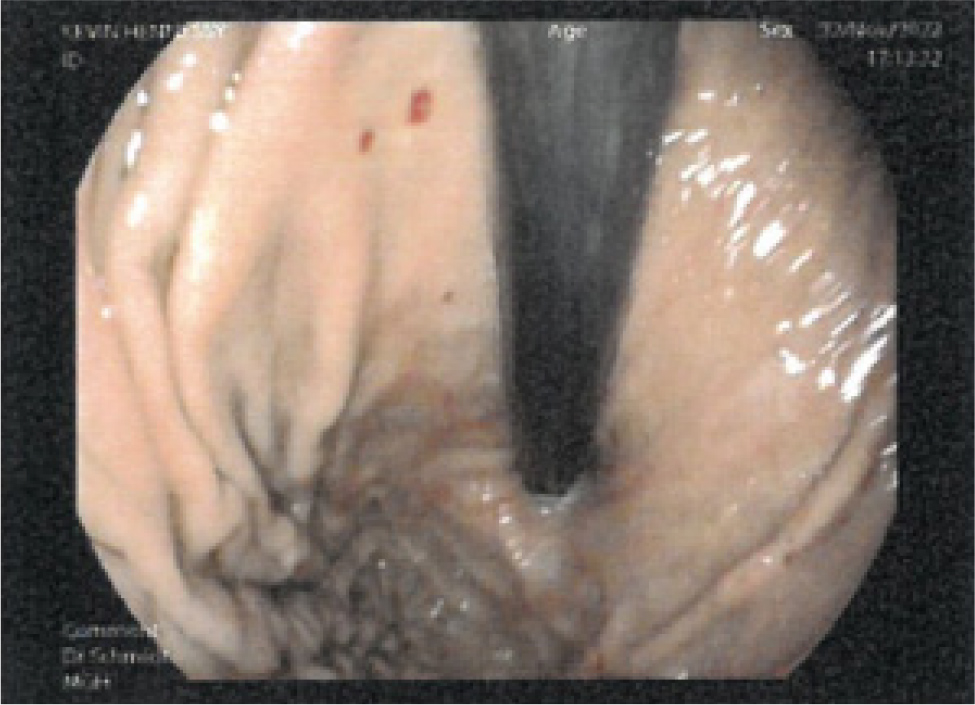
A 47-year-old male was referred to our service with a 3-year history of transfusion-dependent iron deficiency anaemia secondary to known HHT. His HHT was diagnosed by meeting the Curaçao criteria for a definite diagnosis[4]. He had a history of melaena secondary to gastrointestinal tract telangiectasia, epistaxis requiring cauterisation with ENT and pulmonary arteriovenous malformations requiring embolisation with interventional radiology. He had no other past medical history. His mother and brother also have a diagnosis of HHT. Physical examination revealed mucocutaneous telangiectasia; he had a recent oesophago-gastro duodenoscopy and colonoscopy, which confirmed widespread gastrointestinal telangiectasia (Fig. 1 and 2). He previously had argon plasma coagulation, without a significant improvement in his symptoms.
At the time of initial review, the patient reported intermittent melaena. He also reported small volume epistaxis, occurring on average two times per week. The patient denied large volume epistaxis or being aware of swallowing blood during episodes of epistaxis. As a result, it was concluded that his melaena was likely due to gastrointestinal bleeding and not related to epistaxis. At the time of initial review in our clinic, he was being managed with IV iron supplementation, tranexamic acid, and proton-pump inhibitors. In the months prior to starting bevacizumab, his transfusion requirement had ranged from 3–5 units of packed red cells per month to maintain an Hb >8g/dl. His blood tests at the time of initial review in late January 2022 showed a haemoglobin of 8.3 g/dl, ferritin 56.5 ng/mL and a transferrin saturation of 4.6%. An IV iron infusion was arranged.
Our patient consented to bevacizumab therapy. We explained that bevacizumab therapy for HHT is an off-label indication without large trial evidence, and treatment is informed by case reports, case series and expert opinion.
Our patient attended his first bevacizumab infusion in February 2023. His blood tests prior to receiving the infusion showed an Hb of 5.9 g/dl. He reported one small epistaxis in the intervening days and denied any overt gastrointestinal bleeding. We arranged for two units of packed red blood cells in addition to his scheduled bevacizumab. The bevacizumab regimen used was a 5 mg/kg dose given every 2 weeks, for a total of six infusions over a 12-week period. The infusions were given in a day ward setting. Our patient was noted to have a normal white cell count, platelet count, urea and electrolytes, liver function tests, urinary protein, and blood pressure prior to commencing bevacizumab.
He had no history of cardiac disease. He had bloods (full blood count, urea and electrolytes, liver function tests), dipstick urinalysis for protein, and blood pressure checked prior to each infusion. We also monitored his iron studies throughout his treatment.
Our patient had a marked improvement in his symptoms within the first month of treatment. He did not require any further blood transfusion during the three months of treatment. He was given one further IV iron infusion in the final month of his 3-month bevacizumab treatment. He noted a complete resolution of melaena and a significant decrease in epistaxis. His haemoglobin, ferritin and iron studies confirmed this improvement (see Table 1 and Fig. 3); he reported a marked increase in overall well-being and did not report any side effects. Given the significant response to treatment, we have arranged to continue bevacizumab once every three months in an attempt to maintain this treatment response.
DISCUSSION
HHT is diagnosed using the Curaçao criteria[4]. There are four clinical criteria considered to make a diagnosis: first, spontaneous and recurrent epistaxis; second, telangiectasias at characteristic sites; third, visceral arteriovenous malformations or telangiectasias; finally, a first degree relative with HHT. Patients with three or more criteria have a definite diagnosis of HHT; patients with two of the Curaçao criteria have a possible diagnosis of HHT; patients with 0 or 1 of the criteria are unlikely to have HHT. Our patient met all four criteria for a definite diagnosis of HHT.
The rationale for use of anti-vascular endothelial growth factor (anti-VEGF) agents such as bevacizumab is evident when we consider the pathogenesis of HHT. HHT results from genetic variants characterised by loss-of-function mutations in three specific genes: ENG, which encodes endoglin; ACVRL1, which encodes the type 1 receptor ALK1; and SMAD4, formerly known as MADH4, which encodes the transcription factor SMAD4[1]. These genes encode proteins involved in blood vessel formation, and the variants in the genes present in HHT promote angiogenesis. Anti-VEGF drugs such as bevacizumab can be used to try to negate this. Variants in each of the three genes noted above result in different subtypes of HHT[3].
Current evidence for bevacizumab use in the management of GI bleeding secondary to HHT is lacking[1]. The current use of bevacizumab is based on an initial case report in 2008 showing an improvement in high output cardiac failure in a patient with arteriovenous malformations secondary to known HHT[5]. Since then, other case reports and some limited case series have described bevacizumab’s use in GI bleeding in HHT[6,7]. Recently, a small phase 2 trial investigated the effect of bevacizumab on bleeding in HHT in 24 patients[8]. Twelve patients were randomised to bevacizumab and twelve to a placebo. There was a numerical reduction in red cell transfusion requirement, but the trial failed to reach statistical significance. Of note, the trial did not have separate arms for gastrointestinal bleeding versus epistaxis and only two patients with gastrointestinal bleeding were included in the treatment arm. Further descriptions of patient response to bevacizumab in GI bleeding in HHT are needed to further inform current treatment. Key questions on the management of GI bleeding with HHT remain, such as the optimal duration of therapy, whether maintenance bevacizumab is required in patients who have an initial reduction in GI bleeding, and at what dose and frequency this maintenance therapy should be prescribed[6]. The recent phase 2 study of bevacizumab also highlights a high incidence of adverse reactions in both the bevacizumab and placebo arms. This serves as a reminder of the importance of documenting our clinical experience with this emerging treatment option for refractory GI bleeding in patients with HHT, to better inform the safety profile of bevacizumab in patients with HHT[8].
CONCLUSION
Bevacizumab can be an effective treatment option in patients with HHT refractory to traditional management. As seen in this case, it can be effective in reducing blood and iron requirements as well as reducing symptomatic bleeding.