ABSTRACT
Objectives: To illustrate that the protease inhibitor (PI) ritonavir, widely used as part of the treatment for HIV, might cause drug–drug interactions with inhaled corticosteroids.
Material and methods: A case report is presented.
Results: An HIV-positive patient presented with gradually changing body composition that was ascribed to lipodystrophy. Finally, iatrogenic Cushing's syndrome with secondary adrenal insufficiency was diagnosed due to a drug–drug interaction of ritonavir and fluticasone.
Conclusion: Lipodystrophy might mimic Cushing's syndrome. The combination of ritonavir and inhaled fluticasone may lead to systemic steroid excess causing Cushing's syndrome and secondary adrenal insufficiency.
LEARNING POINTS
- The strong drug interaction between inhaled fluticasone and the potent CYP3A4 inhibitor ritonavir can lead to iatrogenic Cushing's syndrome and secondary adrenal insufficiency.
- Lipodystrophy due to antiretrovirals can mimic the diagnosis of Cushing's syndrome.
- Replacing fluticasone or ritonavir is the most important management intervention.
KEYWORDS
Cushing's syndrome, secondary adrenal insufficiency, ritonavir
BACKGROUND
Ritonavir, a protease inhibitor (PI), is commonly used in the treatment of HIV-positive patients to boost levels of other PIs as it is a potent inhibitor of CYP3A4 activity[1]. Because of these pharmacological characteristics, the metabolism of other commonly used drugs may be affected.
Intranasal and inhaled corticosteroids (ICSs) are widely used for suppression of airway inflammation in conditions such as asthma and chronic obstructive lung disease (COPD).
Fluticasone is a substrate of hepatic CYP3A4 and is rarely reported to interact with ritonavir, resulting in steroid accumulation, leading to Cushing's syndrome and adrenal suppression[2]. Detection of iatrogenic Cushing's syndrome is particularly challenging in HIV-positive patients, because the presence of lipodystrophy as a side effect of combination antiretroviral therapy (cART) may mask the diagnosis[3]. This case highlights the strong drug interaction between inhaled fluticasone and low-dose ritonavir, leading to iatrogenic Cushing's syndrome and secondary adrenal suppression. The purpose of this article is to underscore this underreported interaction that may lead to potentially fatal adrenal failure.
CASE PRESENTATION
A 55-year-old man presented in 2013 for a routine check of his HIV-1 infection that had been treated with cART since 2000. His medical history also revealed COPD, hypertension and nephrolithiasis. Over the past years, his cART had been changed several times because of presumptive lipodystrophy and other side effects. His current medication was tenofovir/emtricitabine, atazanavir, ritonavir (100 mg o.d.) and amlodipine.
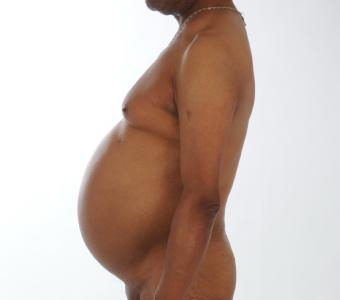
He complained of progressive fatigue, an ongoing predominant rise in his waist circumference and general bone pains. On examination, we saw a healthy man with a blood pressure of 142/90 mmHg. He had pronounced central adiposity and the muscles of the lower extremities were obvious atrophic (Fig. 1).
Figure 1: Pronounced central adiposity and atrophic muscles of the lower extremities in our patient with latrogenic Cushing's Syndrome
We did not notice a moon face or buffalo hump; his appearance had not changed in the previous ten years.
However, based on his complaints and body composition, we considered a diagnosis of Cushing's syndrome. Complementary blood and urinary tests were performed as shown in Table 1.
| Exam | Value | Reference range | Unit |
|---|---|---|---|
| Serum morning cortisol (I) | 18 | 150-700 | nmol/l |
| Serum morning cortisol (II) | 19 | 150-700 | nmol/l |
| Serum ACTH (I) | <5 | 1-60 | ng/l |
| 24-Hours urinary cortisol excretion (I) | 24 | 160-1100 | nmol/l24h |
| 24-Hours urinary cortisol excretion (II) | 28 | 160-1100 | nmol/l24h |
Table 1 - Laboratory and urinary findings
The morning serum cortisol levels were repeatedly extremely low while the adrenocorticotropic hormone (ACTH) level was suppressed. A short synthetic ACTH stimulation test showed a blunted response.
Baseline cortisol level was 31 nmol/l at start with cortisol levels of 66 nmol/l and 83 nmol/l after 30 and 60 min. In a physiological situation, we would aspect a rise of the cortisol level at least above 500 nmol/l. A repeatedly low excretion of cortisol was shown by 24-h urinary examination. A bone density scan revealed skeletal osteopenia. The diagnosis of exogenous Cushing's syndrome with osteopenia and secondary adrenal suppression was made. On inquiry, the patient reported that he had been using intranasal and inhaled fluticasone for over ten years. It had been prescribed by his general practitioner. The strong interaction between the fluticasone and ritonavir resulted in this clinical and biochemical picture. The fluticasone was stopped and the atazanavir and ritonavir were replaced by raltegravir. To prevent adrenal crisis, the patient was put on steroid replacement therapy (hydrocortisone 10 mg o.d. and 5 mg b.i.d.). Six months later, his general condition had improved with normalization of body composition; retrospectively, we concluded that he indeed had had a moon face. Adrenal insufficiency was still present and thus tapering of hydrocortisone was not yet possible.
DISCUSSION
This case highlights the strong drug interaction between fluticasone and ritonavir leading to iatrogenic Cushing's syndrome with osteopenia and secondary adrenal suppression. Only after inquiry we found out that the patient was using inhaled and intranasal fluticasone. Therefore, a thorough medication history is essential in patients with symptoms and clinical features suggestive of Cushing's syndrome. Because fluticasone is widely prescribed for common pulmonary conditions, there is a potential increased risk of developing steroid accumulation in HIV-positive patients on ritonavir-based cART.
The frequent clinical phenotype of lipodystrophy due to antiretrovirals may mask the diagnosis of Cushing's syndrome. Central adiposity and osteopenia are features overlapping with lipodystrophy and Cushing's syndrome, but the presence of peripheral atrophy may be a clue to the presence of Cushing's syndrome[3].
A recent review of the literature did not report cases of iatrogenic Cushing's syndrome in HIV patients using ICSs other than fluticasone[4]. The pharmacokinetics of fluticasone increases the likelihood of systemic steroid accumulation in HIV patients using a ritonavir-based ART regimen. Fluticasone is the most lipophilic ICS and has the longest glucocorticoid receptor-binding half-life. Other ICSs, like beclomethasone and budesonide, may only lead to iatrogenic adrenal suppression and Cushing's syndrome when ritonavir is combined with other potent CYP3A4 inhibitors like clarithromycin or itraconazole[5].
Iatrogenic Cushing's syndrome due to exogenic steroid excess is a clinical diagnosis. Biochemical support comes from the presence of secondary adrenal insufficiency as illustrated by a low morning plasma cortisol and ACTH level. In our case, a synthetic ACTH stimulation test with an absent rise in cortisol finally confirmed the diagnosis. The mainstay of the treatment is to replace ritonavir and change cART and/or substitution of the fluticasone by another local steroid. Tapering of inhaled steroids must strongly be considered to avoid steroid withdrawal symptoms.
CONCLUSION
Fluticasone inhalation therapy may cause iatrogenic Cushing's syndrome with secondary adrenal insufficiency when combined with the strong CYP3A4 inhibitor ritonavir as used in the treatment for HIV. A thorough medication history must therefore be part of the routine management of HIV patients using ritonavir. Low morning cortisol and ACTH levels, low urinary cortisol excretion and blunted response on the synthetic ACTH stimulation test will confirm this diagnosis. Management options include replacing fluticasone and/or ritonavir.