ABSTRACT
A 63-year-old male patient suffered from myalgia, arthralgia cough and erythematous macules, with confluence to the thorax, limbs, thighs and the scrotum. The erythematous macules evolved to blisters with a positive Nikolsky's sign. A cutaneous biopsy revealed satellite cell necrosis. Administration of immunoglobulins resulted in a favourable evolution of the cutaneous lesions. Toxic epidermal necrolysis (TEN) is a rare and potentially fatal mucocutaneous disease. Early recognition, diagnosis and therapy are of the utmost importance.
LEARNING POINTS
- Toxic epidermal necrolysis (TEN) is a rare and potentially fatal mucocutaneous disease.
- TEN requires early diagnosis, appropriate workup and treatment to minimise potential morbidity and mortality.
- Today immunoglobulin therapy is the most accepted form of treatment for TEN.
KEYWORDS
Toxic epidermal necrolysis; Nikolsky's sign; erythematous macules
CASE DESCRIPTION
We present the case of a 63-year-old male patient with a history of psoriasis, type II diabetes mellitus and arterial hypertension. He had been medicated with metformin and methotrexate since 2012, and he had started a treatment with valsartan the month prior to admission.
The patient was admitted to Accident and Emergency department. He had been suffering from constitutional symptoms, myalgia, arthralgia and cough, which had started 5 days before. He also presented non-pruriginous, erythematous macules, which showed a pattern of confluence of the thorax, limbs, thighs and the scrotum. These macules involved more than 30% of his total body surface area.
When the patient was admitted to Accident and Emergency, he was apyretic, with a median arterial blood pressure of 98 mmHg and a heart rate of 92 beats per minute. The results from blood tests done at hospital admission were the following: reactive C protein: 10 mg/dL; BUN: 68 mg/dL; glucose: 180 mg/dL; and bicarbonate: 18 mg/dL.
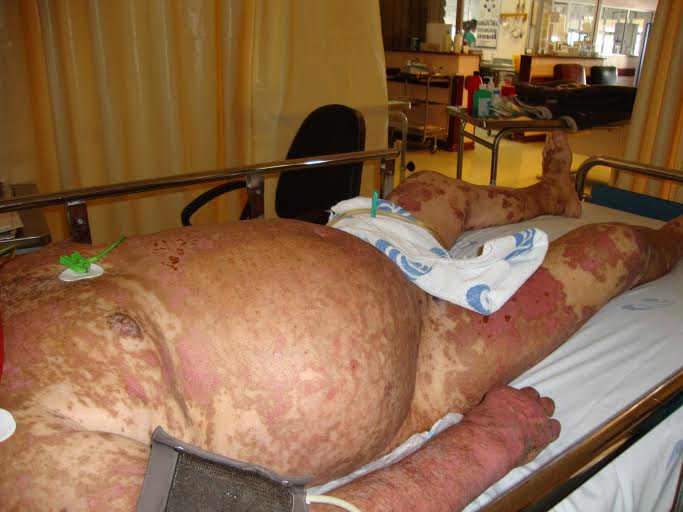
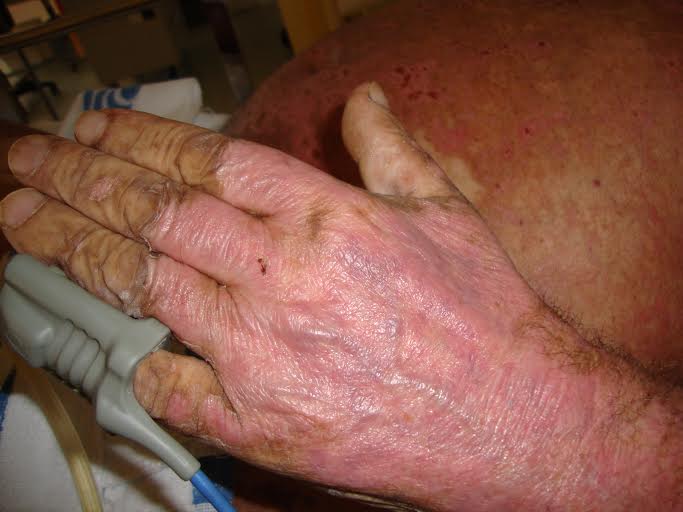
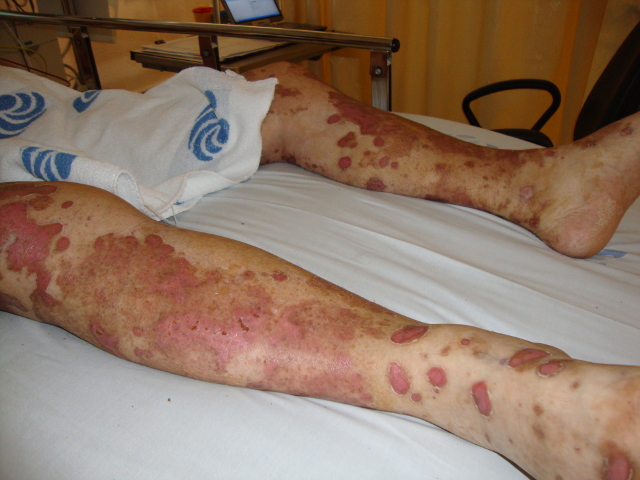
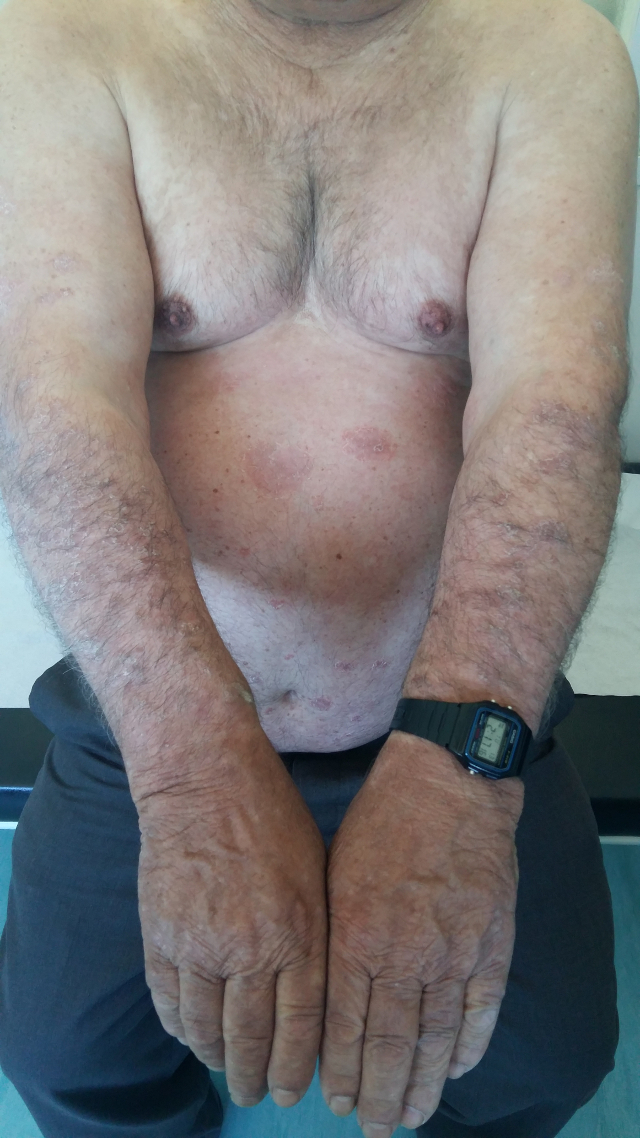
The patient was admitted to the internal medicine department for medical treatment and further study. On the second day after admission, the erythematous macules became violaceous and evolved to blisters with a positive Nikolsky's sign (Figs 1, 2 and 3). Later, several cutaneous areas became eroded, and lesions arose in the oral and genital mucosa, which originated dysphagia and dysuria. The patient was diagnosed with TEN as a probable iatrogenic reaction to the introduction of valsartan. The patient was submitted to a cutaneous biopsy and started a corticotherapy.
Figure 1. Erythematous macules of the thorax, abdomen and limbs, which involved more than 30% of the body surface area.
Figure 2. Desquamative lesions of the hand.
Figure 3. Erythematous macules with a Nikolsky's sign.
On the fifth day, the patient showed signs of sepsis and was transferred to the Intensive Care Unit.
The patient then was given broad-spectrum antibiotics, para-enteric nutrition and high-dose immunoglobulins therapy for 5 days. There was a favourable clinical evolution, with normalisation of inflammatory markers and improvement of cutaneous lesions. An ophthalmology consultation excluded corneal lesion and a urology consultation did not identify any lesion or stenosis of the uro-genital mucosa. Finally, an endoscopy was performed, which did not reveal any stenosis of the upper gastrointestinal tract.
The cutaneous biopsy showed parakeratosis, pyknotic inflammatory cells and satellite cell necrosis in the malpighian layer, thus supporting the clinical diagnosis of TEN. The presence of viral infections (Herpes simplex, Epstein Barr virus and Human immunodeficiency virus) and bacterial infections (mycoplasma) were excluded. Vaccinations had not been administered recently.
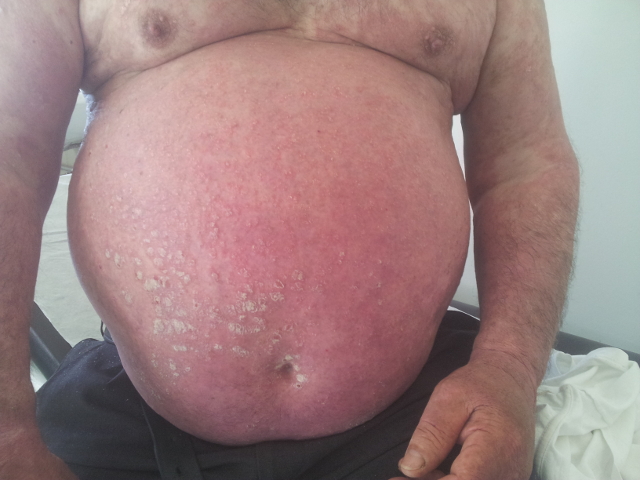
In the last follow-up appointment at 6 months (Fig. 4) and 1 year (Fig. 5) after admission, the patient was in complete clinical remission, maintaining small cutaneous lesions.
Figure 4. Cutaneous scarring and irregular pigmentation of the skin at six months of follow up
Figure 5. Irregular pigmentation of the skin after one year of follow up.
DISCUSSION
TEN is a rare and potentially fatal mucocutaneous disease with an incidence of 0.4-1.2 cases per million[1].
TEN and Steven-Johnson syndrome (SJS) are believed to be variants of the same condition. They are classified based on the skin surface area detached at maximum extent. In SJS, the skin detachment area is inferior to 10% of the body surface area, while TEN is characterized by more than 30% of skin detachment[1].
Incidence increases with age and in some risk groups, such as polymedicated patients, slow acetylator patients and immunosuppressed patients[1].
The exact pathogenic mechanisms of TEN are not completely understood. It is accepted that this disease is a form of delayed hypersensitive reaction[2].
The diagnosis is mainly clinical, based on an exhaustive anamnesis and taking special care in identifying new medication or previous infections.
Initial symptoms can be unspecific and precede cutaneous manifestations by a few days. Typical clinical signs initially include areas of erythematous and livid macules on the skin. The Nikolsky's sign is considered positive when mechanical pressure induces epidermal detachment, but it is not specific for TEN, as it can also be positive in, for example, autoimmune bullous skin diseases[3].
Histological findings of apoptosis of keratinocytes and necrosis of the epidermis in cutaneous biopsies support clinical diagnosis[3].
It is believed that about 80% of all reported cases of TEN are related to adverse reactions to medication, and manifestations develop, on average, 7 to 21 days after the introduction of new medication[1]. Mockenhaupt et al. demonstrated that the risk of developing TEN is higher when new medication is prescribed, and it declines after 8 weeks of administration[4]. Although most clinical studies do not report an association between angiotensin II receptors antagonists and TEN, in our clinical case, valsartan was the only new medication that the patient had taken previously. However, it may also have been the result of a drug interaction between valsartan and either methotrexate or metformin. Common TEN infectious causes were excluded.
TEN manifests itself as a morbiliform rash that evolves to epidermal detachment. Barrier function loss of the epidermis has severe implications on the ability to maintain homeostasis in these patients. Patients are especially prone to bacterial infections[1].
Acute stage management requires a sequential evaluation of the severity and prognosis of this disease, with prompt identification and withdrawal of the culprit drug(s), supportive care and, eventually, a specific drug therapy. In order to evaluate prognosis of TEN patients, the validated SCORTEN disease severity scoring system can be used[3]. Age, presence of malignancy, tachycardia, initial body surface area of epidermal detachment, serum urea, glucose and bicarbonate are used as markers of clinical severity. At admission the patient had a SCORTEN result of 3 (age >40 years, serum urea >28 mg/dl and an initial surface of epidermal detachment >10%), with a predicted mortality of 35.8% [2, 3].
To date, no specific therapy for TEN has shown clinical efficacy in controlled clinical trials. Several treatment modalities may be pursued. In our report, the patient was prescribed a corticotherapy in the first days after admission, and later he was put on an immunoglobulin therapy following admission to the Intensive Care Unit.
The administration of corticotherapy remains controversial, especially after the initial 48hrs, as it compromises healing and contributes to a state of immunosuppression[1, 3]. Today, immunoglobulin therapy is the most accepted TEN treatment. It acts at the level of the FAS receptor and its ligand (FAS-L) is responsible for the apoptosis of the keratinocytes. A 12% reduction of mortality has been demonstrated, necrosis progression is halted and re-epithelization is faster[5].
Mortality rates associated with TEN range between 25 to 35%, and they are closely related to SCORTEN[2, 3]. Most of the time, it is the result of sepsis or multi-organ failure.
More than 50% of patients surviving TEN suffer from long-term sequelae. These include ocular involvement–symblepharon, conjunctival synechiae, cutaneous scarring, irregular pigmentation and persistent erosions of mucous membranes, phimosis, nail dystrophy, and diffuse hair loss[3]. In this clinical case, the patient had cutaneous scarring and irregular pigmentation of the skin. Early recognition, diagnosis and therapy are of the utmost importance.