ABSTRACT
Thyroid stimulating hormone (TSH)-suppressive therapy with levothyroxine is a cornerstone of thyroid carcinoma follow-up therapy, but the achievement of therapeutic goals must be balanced against L-T4 side effects. We describe the case of a 64-year-old cardiopathic patient with papillary thyroid carcinoma and autoimmune thyroiditis, whose cardiac condition worsened during TSH-suppressive therapy. TSH concentrations also fluctuated widely because of changing intestinal absorption due to coeliac disease.
LEARNING POINTS
- TSH-suppressive therapy with levothyroxine (L-T4) to prevent thyroid carcinoma relapse can be a tricky problem in the presence of comorbidities.
- The recent American Thyroid Association guidelines are a useful reference for complex cases of thyroid carcinoma.
- When strict TSH control is crucial, the L-T4 liquid solution may be a valuable tool.
KEYWORDS
Thyroid carcinoma; TSH-suppressive therapy; intestinal malabsorption
CASE PRESENTATION
A 64-year-old woman with coeliac disease and autoimmune thyroiditis was examined 2 years after thyroidectomy with lymphadenectomy for carcinoma (January 2006). The histopathological report described multifocal papillary carcinoma, follicular variant, associated with lymphocytic thyroiditis; the main nodule (6 mm) had infiltrated the thyroid capsule without affecting the fat tissue, vascular or lymphatic vessels (TNM pT1 (G2) pN0 M0). Ten months after surgery, radioiodine remnant ablation had been performed. Subsequent whole body scintigraphy and periodic ultrasound scans were not suggestive of relapse; thyroglobulin concentrations were undetectable but anti-thyroglobulin antibody positivity persisted.
The patient also had ischaemic heart disease, diagnosed on the basis of myocardial scintigraphy as she refused coronarography.
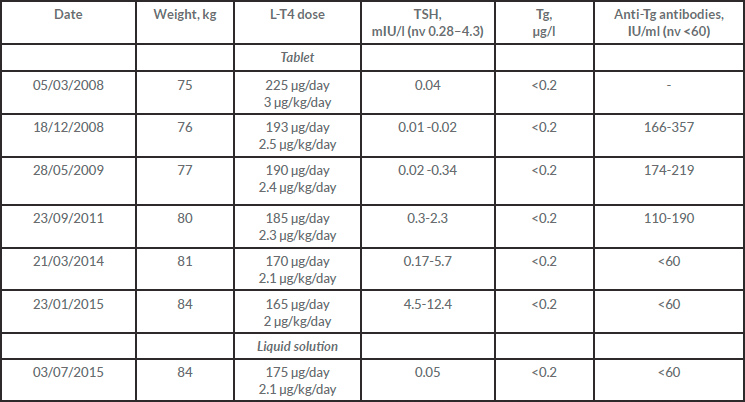
At first examination at our clinic (March 2008), thyroid stimulating hormone (TSH) was suppressed on levothyroxine (L-T4) 3 µg/kg/day, but the treatment was not tolerated due to frequent angina and premature ventricular beats despite beta-blocking therapy. Consequently, L-T4 was tapered to the dose allowing satisfactory control of the side effects (2.4 µg/kg/day). The results of periodic tests are shown in Table 1.
In March 2009, the patient's cardiac condition deteriorated and atrial fibrillation was noted; a CT scan revealed coronary artery disease that required angioplasty. At the same time, the periodic neck ultrasound showed a single 12×7 mm lymph node suspect for local relapse. As a result, TSH inhibition became more pressing even in the absence of significant changes in blood tests or pathological uptake areas at repeated whole body scintigraphy. However, we delayed any L-T4 increase in view of the patient's unstable cardiac condition. Over the following observation period, the L-T4 dosage was frequently modified in response to wide TSH fluctuations, until January 2015 when TSH=12.7 mIU/l was registered. Although the patient declared good compliance with a gluten-free diet except on rare occasions, we suspected problems with absorption and switched from L-T4 tablets to the liquid formulation at the unchanged dose of 2.1 µg/kg/day, and recorded optimal TSH suppression after 6 weeks. Thereafter, follow-up was negative for relapse.
Table 1. Biochemical tests and L-T4 doses
Tg, thyroglobulin; TSH, thyroid stimulating hormone
DISCUSSION
The challenge we describe is not unusual for endocrinologists trying to minimize cancer recurrence risk while also limiting TSH-suppression side effects.
Our patient had been classified as belonging to the high-risk category based on American Thyroid Association (ATA) guidelines[1]. Supra-physiological doses of L-T4 were therefore prescribed as it was mandatory to maintain TSH levels below 0.1 mU/l[1,2] to decrease the risk of recurrence[1–5]. However, the potential benefits of reaching the therapeutic goal must always be balanced against possible harm from subclinical thyrotoxicosis[1,2], a complex undertaking in this case due to the coexistence of cardiac ischaemic and arrhythmogenic disease. The recent ATA guidelines are particularly useful in situations like these, as they give strict indications for the thyrotropin targets, taking both L-T4 side effects and co-morbidities into consideration. According to these guidelines, the recommended TSH target is between 0.5 and 2 mU/l in the presence of specific conditions: an indeterminate response (anti-TG antibodies stable or declining without structural or functional disease), menopause, age >60, and in particular atrial fibrillation[6].
Our patient had coeliac disease, which may mean L-T4 doses need to be increased to reach the target TSH[7–11]. In a retrospective study, Collins et al. demonstrated that the mean initial daily dose of L-T4 needed to reach euthyroidism was higher in patients with untreated coeliac disease and hypothyroidism than in those with hypothyroidism alone[12].
In our patient, switching from tablets to the liquid formulation overcame the negative effect of coeliac disease on drug absorption, producing more stable TSH concentrations with lower L-T4 doses; this effect is connected with the intrinsic characteristics of the drug. Levothyroxine is a lipophilic molecule that is absorbed in the upper intestinal tract. The tablet formulation contains a stable salt and excipients, which must be dissolved at the gastric acid pH to convert sodium L-T4 into a lipophilic molecule[13]; this will reach the duodenum and jejunum, where around 70% of the tablet L-T4 content will be absorbed[14–16]. The liquid formulation contains only L-T4 dissolved in a lipophilic solution of glycerine and ethanol; L-T4 also maintains its stability when added to breakfast beverages[17,20].
In an in vivo study comparing the three available formulations of L-T4 (tablet, soft gel capsule, liquid solution), the liquid solution showed the best pharmacokinetics indices and the fastest blood peaks[18,19,21]. Brancato et al. reported a significant decrease in serum TSH levels in 68% of patients switched from tablets (taken according recommendations) to the liquid solution at the same dose. Interestingly, the patients in whom serum TSH decreased had a greater prevalence of factors known to interfere with L-T4 intestinal absorption (gastrointestinal diseases or drugs)[22].
CONCLUSION
Achieving a balance between therapeutic goals and the side effects of L-T4 is a thorny issue, especially when comorbidities are present. The oral solution is a valuable tool in complex cases where strict control of therapeutic goals is crucial.