ABSTRACT
AFIP1L1-PDGFRA fusion can only be confirmed through molecular and cytogenetic investigations causing a delay in the diagnosis. However, patients with this mutation need urgent treatment because they present hypereosinophilia which may be associated with short-term tissue damage. Thromboembolism is a known cause of death in hypereosinophilic syndrome. A case of Loeffler endocarditis due to FIP1L1-PDGFRA-associated chronic eosinophilic leukemia presenting hemiparesis with fever, which also mislead the initial diagnosis, is reported.
LEARNING POINTS
- FIP1L1-PDGFRA fusion occurs in 10% of patients with idiopathic hypereosinophilia.
- Thromboembolism is a known cause of death in hypereosinophilia.
- The prognosis of FIP1L1-PDGFRA-associated chronic eosinophilic leukemia has been profoundly changed by imatinib treatment.
KEYWORDS
Hypereosinophilia, stroke, cytomegalovirus, Loeffer endocarditis
INTRODUCTION
Hypereosinophilia has generally been defined as a peripheral blood eosinophil count greater than 1.5 x109/l and may be associated with tissue damage. The classification of eosinophilic diseases was revised in the 2008 World Health Organization scheme of myeloid neoplasms and a new major category was created: "Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGRFA/B or FGFR1". Moreover, in 2011 the Working Conference on Eosinophil Disorders and Syndromes proposed a new terminology for eosinophilic syndromes in which hypereosinophilia subtypes were divided into a hereditary variant, an undetermined significance (idiopathic), a primary (clonal), and a secondary (reactive) subtype. Each subtype associated with organ damage is referred to as hypereosinophilic syndrome (HES). The HES incidence rate was 0.036 per 100,000 and FIP1L1-PDGFRA fusion occurs in 10% of patients with hypereosinophilia[1]. The most common presenting symptoms are weakness, cough, dyspnea, myalgias, or angioedema, rash, and fever. Essentially all organ systems may be susceptible to the effects of sustained eosinophilia. Endocardial damage with resulting platelet thrombus can lead to mural thrombi and increased embolic risk. Thromboembolism is a known cause of death in HES (13%)[1]. Imatinib is considered to be the definitive treatment for PDGFRA/B-rearranged. The goal of the therapy is to mitigate eosinophil-mediated organ damage. Anticoagulants and anti-platelet agents have demonstrated variable success in preventing recurrent thromboembolism. Cardiac surgery can extend the life of patients with late-stage heart disease manifested by endomyocardial fibrosis, mural thrombosis, and valvular insufficiency. In fact, endomyocardectomy for late-stage fibrotic heart disease can improve cardiac function[1].
CASE PRESENTATION
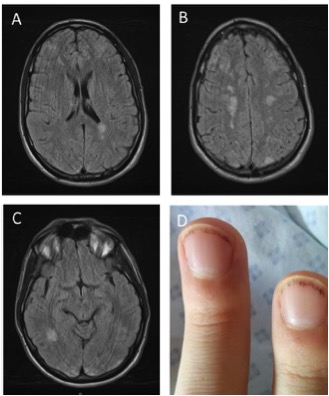
A 26-year-old male with a history of post traumatic splenectomy was admitted to the emergency department for headache and fever. He had not been treated. Physical examination showed left hemiparesis and distal lesions on the nails (Fig. 1). He was transferred to the intensive care unit.
The authors initially considered the following differential diagnoses: infectious encephalitis and auto-immune encephalitis but these were excluded after the diagnostic investigations described below.
Cerebral magnetic resonance imaging (MRI) revealed multiple cerebral emboli (Fig. 1). Echocardiography did not show intracardial thrombus valvulopathy or vegetation. However, heart MRI detected endomyocardial fibrosis with severe impairment of the papillary muscles.
The patient had increased C-reactive protein (73mg/l). Lumbar puncture was normal. Bacterial cultures remained sterile. Blood tests showed 20 G/L eosinophils and 8 G/L lymphocytes. Bone marrow puncture revealed a marked increase in eosinophilic cells (20%) with lymphocyte T but no evidence of atypical cells. Transient T-cell monoclonal lymphocytosis was found but without increase in interleukine 5 plasma level.
The autoimmunity study revealed negative titers for anti-double-stranded deoxyribonucleic acid and for antinuclear and antineutrophil cytoplasmic antibodies (ANCA). Screening for thrombophilic disorders was positive (antiphospholipid antibodies) as was testing for EBV and CMV serology.
Finally, a rearranged platelet-derived growth factor receptor alpha was identified . A diagnosis of stroke complicating Loeffler endocarditis due to Pdgfra-associated chronic eosinophilic leukemia and primary CMV infection with transient antiphospholipid antibodies was made. The patient was treated by imatinib and corticosteroids and was discharged from the ICU to a center for stroke rehabilitation with normalization of his eosinophil blood count.
Corticosteroids were discontinued one month later. Antiphospholipid antibodies test was negative six months later.
Figure 1. Figure 1. Panel A, B, & C: Cerebral magnetic resonance imaging (MRI) revealed multiple cerebral emboli. Panel D: Splinter hemorrhage on nails
DISCUSSION
Patients with FIP1L1-PDGFRA mutation need urgent treatment because hypereosinophilia may be associated with short-term tissue damage[1]. A case of Loeffler endocarditis due to FIP1L1-PDGFRA-associated chronic eosinophilic leukemia presenting hemiparesis and also fever misleading the initial diagnosis is presented in this paper. Fever was caused by primary CMV infection. Overall a high level of EBV and CMV IgM cross reactivity already demonstrated indicating that serology is unreliable in these diagnoses[2]. Primary CMV infection was confirmed by PCR in this case. Antiphospholipid antibodies have been described in primary CMV infection and recent studies highlight the risk of arterial thrombosis in primary CMV infection[3].
Our patient presented high risk of thrombosis due to both the primary CMV infection and the Loeffler endocarditis due to Pdgfra-associated chronic eosinophilic leukemia. Transient T-cell monoclonal lymphocytosis was due to primary CMV infection but did not cause hypereosinophilia because it did not increase interleukine 5 plasma level.
In the case of hypereosinophilia, the first diagnostic step is to exclude secondary causes of eosinophilia: infections, atopy, drug reaction, collagen-vascular disease, etc. The second step is to evaluate the patient for primary eosinophilia by examining the blood smear and the bone marrow aspiration, and by immunophenotyping (aberrant T-cell population with cytokine-production).
The patient was successfully treated by imatinib. Although in-depth and durable molecular responses occur with imatinib, discontinuation of the drug can lead to relapse. Imatinib can effectively suppress but not eliminate the FIP1L1-PDGFRA clone in most patients. In contrast to chronic myeloid leukemia, very few cases of acquired imatinib resistance have been reported, and most cases have occurred during the blast phase of disease with a resistance to all of the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib.