ABSTRACT
This case demonstrates the therapeutic challenges encountered when managing an acute pulmonary embolism in a cancer patient with thrombocytopenia. A 64-year-old man with a history of lung cancer receiving chemotherapy was admitted to Walsall Manor Hospital with haemodynamic instability consistent with a pulmonary embolism, proven on computed tomographic pulmonary angiogram. His platelet count was noted to be 35×109/l (chemotherapy-induced thrombocytopenia). After discussions, he was deemed not suitable for thrombolysis based on risk versus benefits. The patient was initially transfused one adult dose of platelets and treated with half the therapeutic dose of low molecular weight heparin (LMWH). The same management plan was followed until the platelet count exceeded 50×109/l, after which the patient was established on the full therapeutic dose of LMWH. Clinically, the patient improved and was discharged. Three months after discharge, follow-up revealed sustained clinical improvement while the patient continued to be on the full therapeutic dose of LMWH with a stable platelet count.
LEARNING POINTS
- Cancer patients have a three-fold higher risk of venous thromboembolism compared with non-cancer patients, but also a higher risk of bleeding, hence neoplasm is considered an absolute contraindication to thrombolysis by the European Society of Cardiologists.
- The management of an acute pulmonary embolism in cancer patients with thrombocytopenia is still debated. However, a few recognised medical societies and expert opinions have established recommendations on this specific area, such as the British Committee for Standards in Haematology, the American Society of Clinical Oncology and the International Society of Thrombosis and Haemostasis.
- Expert opinion agrees on: giving the full therapeutic dose of low molecular weight heparin (LMWH) if the platelet count is above 50×109/l; if it drops below 50×109/l, halving the dose of LMWH with or without platelet transfusion until the platelet count improves above 50×109/l; and when the platelet count is below 20–30×109/l, withholding anticoagulation and considering the insertion of an inferior vena cava filter.
KEYWORDS
Pulmonary embolism, PE, venous thromboembolism, VTE, cancer, thrombocytopenia
CASE DESCRIPTION
A 64-year-old man presented with acute-onset shortness of breath worsening dramatically over 24 hours in the absence of cough, chest pain or haemoptysis.
The patient had a background history of hypertension, osteoarthritis and lung cancer (squamous cell carcinoma stage T4N2M1a) for which he had been treated with chemotherapy (gemcitabine and cisplatin). He was known to subsequently have chemotherapy-induced thrombocytopenia. His performance status was zero.
He had previously had a pulmonary embolism (PE) 2 months before this current admission where he had received the full therapeutic dose of low molecular weight heparin (LMWH) for only 14 days after which it was stopped due to severe thrombocytopenia. Repeat computed tomographic pulmonary angiogram (CTPA) at that time had demonstrated a normal pulmonary angiographic scan. There was no family history of malignancies, haematological disorders or venous thromboembolism (VTE).
On examination, the patient was alert and well orientated but not able to complete one full sentence with a respiratory rate of 28 breaths per minute. His oxygen saturation level was 91% on room air, and his temperature was 36.5°C. Chest examination revealed a central trachea with reduced air entry over the right lower hemi-thorax which was dull to percussion. Cardiovascular examinations revealed tachycardia with a heart rate of 114 beats per minute which was regular in nature, and blood pressure of 103/70 (the last blood pressure recorded in the outpatient department was 130/78). Abdominal examinations were not significant. Both calves were of equal size, soft and non-tender
METHODS AND PROCEDURES
Initial investigations on admission included: arterial blood gas which demonstrated type 1 respiratory failure (PaO2 6.7 kPa) with normal pH, PaCO2, electrolytes and lactate. An electrocardiogram showed sinus tachycardia at 114 beats per minute with an S1Q3T3 pattern. Chest x-ray showed right middle and lower zone patchy changes, with no new changes compared to previous x-rays (right-sided lung cancer). Venous bloods showed thrombocytopenia (platelet count of 35×109/l, that had been fluctuating as per chemotherapy cycles), normal haemoglobin and white cell count, and mildly raised C-reactive protein. Otherwise the clotting profile was normal with normal kidney and liver functions.
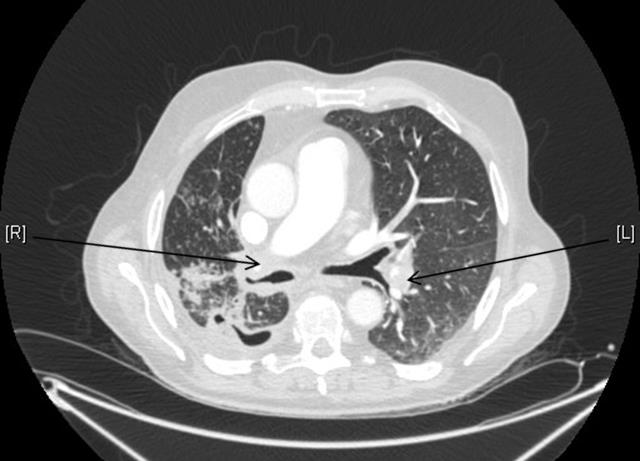
Three hours into the patient’s admission, he required more oxygen (8 l/min) and his blood pressure dropped to 93/55 from 103/70 mmHg. He had an urgent CTPA, which confirmed multiple PEs in the left pulmonary artery and its branches with the tumour occluding the right pulmonary artery (Fig. 1). Ultrasound Doppler was performed and confirmed deep vein thrombosis (DVT) in both the right and left popliteal veins.
Figure 1. Computed tomographic pulmonary angiogram. (R) refers to the right pulmonary artery which is occluded by a tumour. (L) refers to multiple pulmonary emboli in the left pulmonary artery branches
Once the diagnosis of PE was confirmed on the CTPA, the patient was discussed with the out-of-hours medical consultant who advised discussing the thrombolysis option with the patient. The Intensive Care Unit team were involved, at which point the patient’s blood pressure improved, therefore removing the indication for thrombolysis due to the greater chance of bleeding than of benefit.
The patient was observed closely until seen by the on-call consultant on the morning ward-round, who reinforced the difficulties of treating acute PE in thrombocytopenia. The patient agreed to have half the dose of LMWH. The case was discussed with the on-call haematologist and oncologist regarding further management and anticoagulation.
The repeated platelet count dropped to 26×109/l and the oncology team on-call reviewed the patient and suggested not giving LMWH at this stage until the platelet count improved.
However, the patient continued to improve clinically without any anticoagulation treatment (36 hours into his admission) and remained haemodynamically stable requiring no oxygen therapy.
On day two of admission, the platelet count dropped to 25×109/l. The patient was discussed with the haematologist on-call who suggested giving one adult dose of platelets with half the therapeutic dose of LMWH, and advised the platelet count should be monitored every 12 hours and the case be re-discussed with the haematologist for further advice.
On day three, the repeated platelet count improved to 40×109/l. Further Haematology input suggested another adult dose of platelets with half the therapeutic dose of LMWH as before. The patient was reviewed by the oncologist again who agreed to continue with half the therapeutic dose of LMWH until the platelet count improved to more than 50×109/l.
On day four, the platelet count was noted to be 64×109/l. The haematologist advised commencing a full therapeutic dose of LMWH with regular monitoring of factor Xa levels to ensure that the patient was over-coagulated.
On day five, the patient had clinically and biochemically improved with the platelet count noted to be 80×109/l. The patient was discharged home on a full therapeutic dose of LMWH with a plan to repeat his platelet count in the community in 1 week’s time and to be reviewed in the oncology outpatient clinic in 2 weeks following discharge.
OUTCOME AND FOLLOW UP PLAN
The patient was reviewed by the oncologist in the outpatient clinic 2 weeks after his discharge, and continued to improve. Overall, the patient had completed two and a half cycles of gemcitabine and cisplatin instead of the initially proposed four-cycle course. The oncology team decided that the patient should not receive any more chemotherapy at this stage due to the radiological improvement in cancer size as well as his increased risk of both thrombocytopenia and recurrent VTEs if chemotherapy were continued.
The patient then was followed up 3 months after discharge. He continued to improve while on the full therapeutic dose of LMWH. He exhibited no symptoms or signs suggestive of a recurrent VTE and had been showing a consistently stable platelet count since his discharge. The plan is to continue with the full therapeutic dose of LMWH until he is reviewed in the oncology outpatient clinic in 6 months’ time.
DISCUSSION
Cancer patients have a higher risk of developing VTEs as well as thrombocytopenia than non-cancer patients[1,2]. According to the European Society of Medical Oncology, cancer patients have a three-fold higher risk of PE compared with non-cancer patients. Also, cancer patients receiving chemotherapy have a seven-fold higher risk of PE compared with non-cancer patients[2].
Thrombocytopenia is defined as a platelet count of less than 100×109/l and in cancer patients is usually attributed to bone marrow suppression. The incidence rate of thrombocytopenia in cancer patients varies according to different factors including type of cancer (solid organ or blood cancer), use of chemotherapeutic agents or drugs with a known risk of thrombocytopenia (such as heparin), and co-existing haematological disorders or other co-morbidities such as sepsis, etc.[1,3]. Based on these factors, the estimated rate of thrombocytopenia in cancer patients can vary between 21% and 70%
[4].
In cancer patients with a low bleeding risk, different international organisations (the American Society of Clinical Oncology (ASCO), International Society of Thrombosis and Haemostasis (ISTH) and British Committee for Standards in Haematology (BCSH)) have established recommendations on the management of VTE and its secondary prevention[5,6]. Strong evidence has been found to support the use of extended anticoagulation therapy if the bleeding risk is not high[1,5,6]. LMWH is recommended as a drug of choice when treating an acute VTE episode in cancer patients [1, 5–8]. The lack of randomised controlled trials means the use of the novel oral anticoagulants (NOACs) for either the prevention or the treatment of VTE in patients with cancer is not recommended until more data become available[5].
On the other hand, it is still not clear how VTE should be managed in cancer patients with a high bleeding risk (e.g. concomitant thrombocytopenia). A literature review was carried out using MEDLINE, EMBASE, CINAHL, NICE Evidence, The Cochrane Library and a general web search. Some recommendations have been established based on expert opinion among the panel members of well-recognised medical societies as summarised below.
ISTH guidance was established to help clinicians managing challenging cases of cancer-associated VTE (including proximal DVTs and segmental or more proximal artery PEs) in those with concomitant thrombocytopenia. The ISTH guidance emphasised the importance of dealing with such patients on an individual basis, accounting for variable factors including the possible aetiology of the thrombocytopenia, its severity and expected duration, risk factors for bleeding as well as patient preference[9]. The ISTH consensus guidance agreed on a platelet count of 50×109/l as the empirical cut-off for platelet transfusion when managing patients with cancer-associated VTE and thrombocytopenia. They strongly recommended the use of full therapeutic doses of anticoagulation without platelet transfusion in patients with cancer-associated VTE and a platelet count of more than 50×109/l. When the platelet count drops below 50×109/l, full therapeutic doses of anticoagulation with platelet transfusion is recommended until the platelet count reaches the empirical cut-off (i.e. 50×109/l)[9]. If platelet transfusion is contraindicated, the ISTH suggests (with weak evidence) the insertion of a retrievable inferior vena cava filter and its removal when the platelet count recovers after which anticoagulation is to be resumed[7,9].
The BCSH recommends the use of a full therapeutic dose of LMWH to treat established VTE in cancer patients with a platelet count above 50×109/l, while half the dose of LMWH is recommended when the platelet count is between 25×109/l and 50×109/l. It is recommended that anticoagulation therapy be withheld when the platelet count is below 25×109/l[6].
ASCO has identified a platelet count cut-off when withholding therapeutic anticoagulation in cancer patients with VTE should be considered. ASCO recommends that severe thrombocytopenia (platelet count below 20×109/l) is an absolute contraindication to therapeutic anticoagulation, while a platelet count of less than 50×109/l is considered a relative contraindication[5]. Unfortunately, no further details are provided regarding the alternative therapeutic options for these patients.
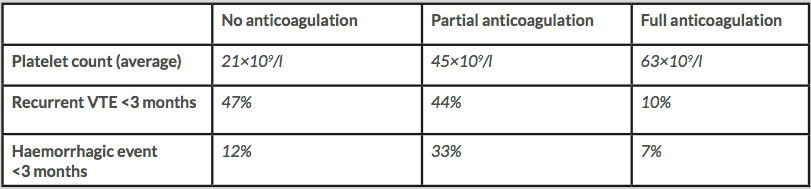
The literature review revealed one retrospective study by Kopolovic et al. that estimated the risk of a haemorrhagic event versus VTE recurrence after treating acute VTE in 74 cancer patients with associated thrombocytopenia[1]. VTE episodes included PEs, DVTs or thromboses in other venous systems. Based on the severity of the thrombocytopenia, three main approaches were followed: no anticoagulation therapy, partial treatment with LMWH (using a prophylactic dose, a 50–75% therapeutic dose or a shorter therapeutic duration), or full-dose anticoagulation with or without platelet transfusions. Their results are summarised in Table 1.