ABSTRACT
A 56-year-old woman presented with cognitive impairment, confusion and slowed speech, muscle cramps and peripheral paraesthesia preceded by vomiting. Blood tests revealed severe hypokalaemia, hyponatremia, hypomagnesemia and hypocalcaemia. Following a diagnosis of Takotsubo cardiomyopathy based on ultrasonography, the patient was treated with electrolyte supplementation and recovered within 48h. When heart failure is suspected, electrolyte abnormalities should be carefully ruled out as they can affect cardiac function.
LEARNING POINTS
- The association between electrolyte abnormalities and Takotsubo cardiomyopathy has still not been well established in the literature.
- Hypomagnesemia and hypocalcaemia can contribute to cardiac akinesia and so should be ruled out in heart failure.
- Correction of hypomagnesemia and hypocalcaemia is an important and an under-estimated part of the optimal treatment of cardiac failure.
KEYWORDS
Takotsubo cardiomyopathy, hypomagnesemia, hypocalcaemia, heart failure
CASE DESCRIPTION
A 56-year-old woman was admitted to the emergency department because of cognitive impairment, confusion and slowed speech, muscle cramps and peripheral paraesthesia lasting a few days. The patient’s family reported very poor food intake in preceding months, and some episodes of vomiting after eating during the previous few days.
The patient’s medical history was negative for both somatic and psychiatric disorders. The only medication she was taking was a PPI. She had been smoking cigarettes since adolescence (40 pack-years).
Blood tests revealed severe hypokalaemia (1.7 mmol/l), hyponatremia (120 mmol/l), hypomagnesemia (0.35 mmol/l) and hypocalcaemia (ionized calcium 0.80 mmol/l), so the patient was admitted to the intensive care department for electrolyte supplementation. Complete neurological recovery was achieved in 48 h after partial correction of electrolyte anomalies.
During the following days, we treated progressive cardiac failure with non-invasive ventilation and the administration of diuretics.
CASE DESCRIPTION
A 76-year-old female patient with a medical history of paroxysmal atrial fibrillation and depression was hospitalised on 10 January 2016 due to a pulmonary thromboembolism. During hospitalisation, the patient started sertraline 100 mg daily, rivaroxaban 20 mg daily, and furosemide 10 mg twice daily. Fifteen days after the start of therapy, the patient developed a rash characterised by urticarial plaques on her back, upper limbs, and anterior surfaces of her thighs. In addition, tense, sero-haemorrhagic blisters developed on both legs (Figs. 1 and 2). No lesions were observed on mucosae.
The patient was followed in the Internal Medicine and Dermatology Service. The tentative diagnosis was that of a bullous pemphigoid secondary to drug use or neoplastic aetiology. Given the known association between furosemide and bullous pemphigoid, its use was discontinued and the patient started on therapy using a topical corticosteroid, betamethasone cream, twice a day.
Methods and Procedures
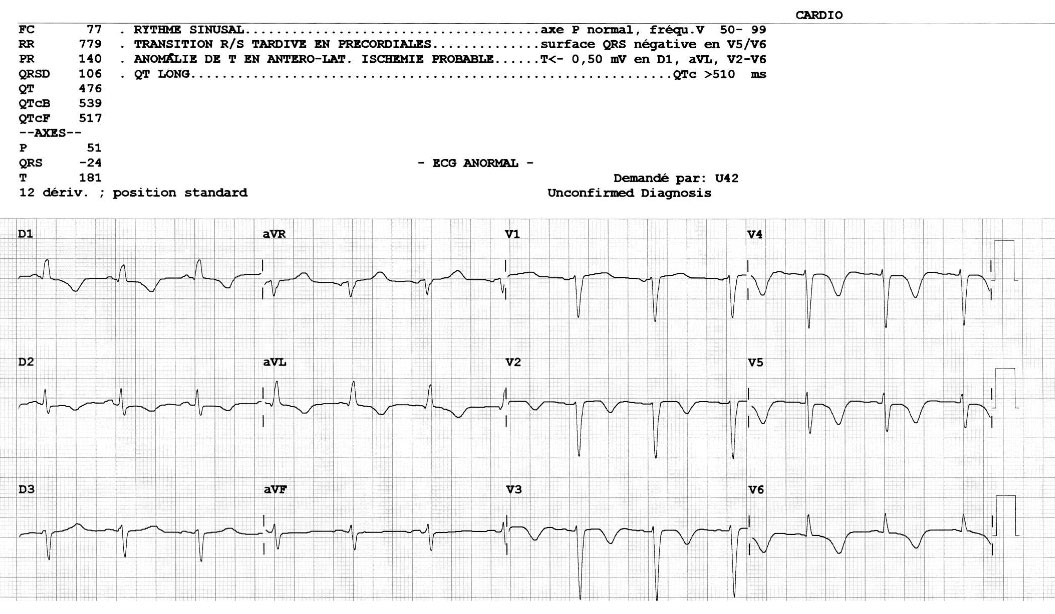
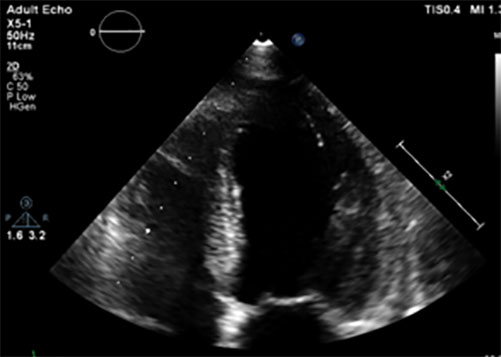
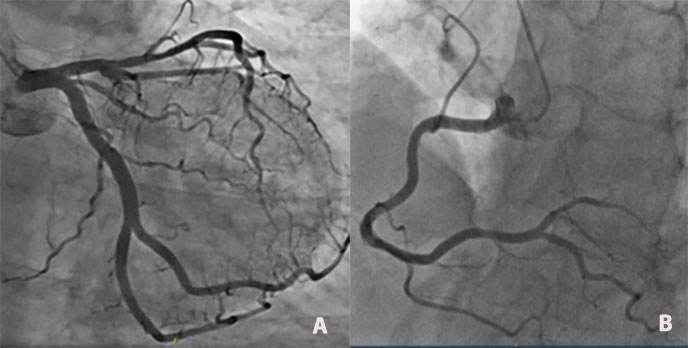
A cardiac ECG revealed diffuse negative T waves in the precordial and lateral leads without chest pain (Fig. 1). Troponin T HS was raised (417 ng/l, normal value <15) and proBNP was 35,000 pg/ml (normal value <125 pg/ml). Cardiac echography showed diffuse septo-apical akinesia with an ejection fraction of 25–30% (Fig. 2). A diagnosis of Takotsubo cardiomyopathy (TC) was made based on ultrasonography results and confirmed by the finding of no significant lesions in the coronary arteries at coronarography (Fig.3 a-b) A second heart ultrasonography, 10 days later, showed global improvement in ventricular function (FEVG 55%) and disappearance of the diffuse akinesia.
Figure 1. ECG showing diffuse negative T waves in the precordial and lateral leads.
Figure 2.Cardiac ultrasonography showing diffuse septo-apical akinesia with global ejection fraction impairment (4-chambers view).
Figure 3 (a). Selective left coronary angiogram showing no significant lesions. (b) Selective right coronary angiogram showing no significant lesions.
There was no recurrence of either the hyponatremia or the hypokalaemia, which were probably caused by the vomiting, or of hypomagnesemia, which resolved after supplementation and stopping the PPI. Hypocalcaemia was probably secondary to severe hypomagnesemia. Further investigations ruled out the presence of a pheochromocytoma.
DISCUSSION
Hyponatremia, hypokalaemia and hypomagnesemia are common abnormalities caused by heart failure, as electrolyte homeostasis is affected by the neurohormonal responses to low cardiac output and by medication for heart failure (in particular diuretic therapy).
Hyponatremia appears to be an independent predictor of increased mortality[1], while hypokalaemia is correlated with sudden cardiac death[2].
Reciprocally, cellular contraction mechanisms can be influenced by electrolyte abnormalities and impact on cardiac function and promote heart failure. Current heart failure guidelines do not highlight the importance of correcting hypomagnesemia and hypocalcaemia which possibly contribute to failure of the heart’s pumping action.
Hypocalcaemia has been related to reversible heart failure which was treated with supplementation, although the mechanism is not well understood. A recent case report associated hypocalcaemia with diffuse TC-like akinesia[3].
Recognised as a predisposing factor for malignant ventricular arrhythmias, hypomagnesemia has also been associated with the development of mitochondrial alterations leading to myocardiocyte death and hypercoagulability. Moreover, magnesium directly provokes myocardial vasodilation, indirectly affecting cardiac contractility[4].
Our patient presented with new ECG abnormalities, troponin elevation, and wall motion abnormalities that extended beyond the territory of a single artery, without coronary artery occlusion. This clinical presentation corresponds with TC, which we attributed principally to electrolyte abnormalities.
The most common TC triggers described in the literature are emotional stress (fear, grief), neurological events (cerebrovascular accidents), invasive procedures, drug administration (dobutamine, SSRI) and, recently, electrolyte abnormalities.
Catecholamine-induced cardiotoxicity and microvasculature dysfunction are considered the most common causes of TC[5].
The following points should be kept in mind:
- Electrolyte disorders (as reported for hypomagnesemia) may provoke the microvascular dysfunction believed to cause TC.
- A cellular mechanism related to electrolyte disorders can contribute to cardiac impairment, as can occur in hypocalcaemia.
- An electrical abnormality could induce a neurohormonal stress reaction, while a neurovegetative mechanism could provoke heart failure.
- Akinesia persists for a while after a trigger has been removed because of the phenomenon of myocardial stunning.
- TC describes a group of heterogeneous conditions whose physiopathology probably slightly differs from each other.
Further research into TC physiopathology, the impact of electrolytes on cellular function and the possible impact of cellular dysfunction on neurohormonal response is required.
This case report underlines the link between electrolyte disturbances and wall motion abnormalities beyond the well-known pro-arrhythmogenic effects of electrolyte abnormalities.
Consequently, in clinical practice, when heart failure is suspected, electrolyte abnormalities should be carefully ruled out because of their possible triggering role and, if present, should be corrected as an essential part of cardiac treatment.