ABSTRACT
We describe the case of a patient with malignant vasodepressive cough syncope. We demonstrated a vaso-vagal mechanism related to left vagal neuritis, by means of laryngoscopy and laryngeal electromyography. The condition resolved with steroid therapy.
LEARNING POINTS
- Left vagal neuritis should be considered in the differential diagnosis of recent onset repetitive loss of consciousness, in particular if cough related.
- Steroids were used to successfully treat recent onset cough-related syncope.
- Relatively simple trials of drug therapy can sometimes avoid intensive investigations; in our case, the use of a systemic steroid would probably have avoided many radiological and endoscopic examinations.
KEYWORDS
Syncope, cough, vagal, neuritis
CASE REPORT
A 74-year-old man was admitted to the emergency department with paroxysmal dry cough attacks complicated by traumatic syncope. He had coronary artery disease with previous angioplasty of the circumflex coronary artery, had a dual-chamber pacemaker for atrio-ventricular block, and was on anticoagulant therapy for paroxysmal atrial fibrillation. Medications included apixaban 5 mg twice daily, enalapril 5 mg once daily, metoprolol 25 mg twice daily, and atorvastatin 20 mg once daily.
At presentation, blood pressure and heart rate were normal, the patient did not have fever or altered mental status, and clinical examination was unremarkable. Chest x-ray excluded parenchymal consolidation, obstructive pulmonary disease and signs of heart failure, and revealed only mild left diaphragm elevation. The electrocardiogram showed sinus rhythm with first-degree atrio-ventricular block. Pacemaker interrogation and the echocardiogram were normal.
Methods and procedures
The patient was admitted to the general medicine department, where he was first treated with antibiotics, cough sedatives and bronchodilators without any improvement; he still experienced up to 20 syncopal episodes per day with jerking movements preceded by cough. Enalapril was stopped. Thorax computed tomography (CT) excluded parenchymal disease or pulmonary embolism.
A bronchoscopy was performed to explore other causes of cough, but cytology, fungal antibody testing and bacterial cultures were all negative. Neurological assessment with a cerebral CT scan, cerebral magnetic resonance imaging (MRI) and supra-aortic trunks Doppler were also negative. The electroencephalogram showed slowing of cerebral waves during jerking movements without critical activity. Laryngoscopy was performed and showed signs of chronic sinusitis. Esophagogastroduodenoscopy (EGDS) did not reveal any signs of gastroesophageal reflux or other pathology.
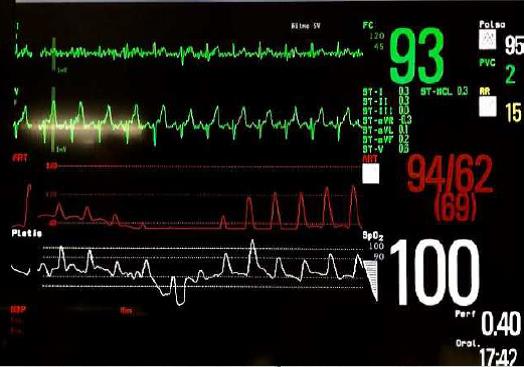
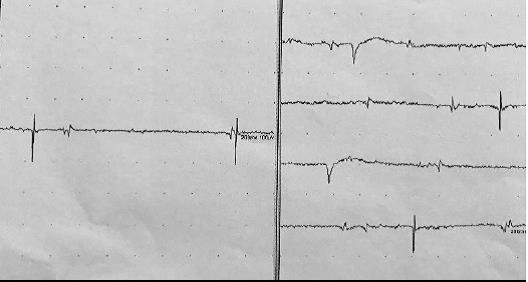
During hospitalization and therapy with enoxaparin, the patient developed haemorrhagic shock due to right shoulder post-traumatic haematoma (haemoglobin fell from 16 to 7 g/dl) with episodes of supraventricular tachycardia. He was transferred to the cardiovascular intensive care unit where he was treated with fluids and administration of blood components resulting in haemodynamic and rhythm stabilization. Over the next few days he presented several new syncopal attacks. During these episodes, invasive pedidial blood pressure monitoring demonstrated a significant drop in blood pressure without changes in heart rate (Fig. 1). To investigate whether syncope was related to an abnormal vagal reflex, atropine 1 mg i.v. was administered as vagolytic treatment, resulting in temporary control of syncopal episodes. Another otolaryngologist assessment with laryngoscopy revealed hypomobility of the left vocal cord. In order to determine if vagal nerve dysfunction was present, laryngeal electromyography was performed and revealed signs of acute neurogenic damage of the cricothyroid and thyroarytenoid left muscles with denervation potentials (Fig. 2).
Figure 1. Invasive blood pressure monitoring through the pedidial left artery, avoiding jerk-related artefacts during cough, demonstrated a significant drop in blood pressure without changes in heart rate during the syncope attack
(baseline arterial pressure 125/80 mmHg; immediate post-cough arterial pressure 60/30 mmHg)
Figure 2. Laryngeal electromyography revealed denervation potentials of the cricothyroid and thyroarytenoid left muscles
Once organic causes were excluded through imaging, under the hypothesis of vagal neuritis we started methylprednisolone 40 mg bid i.v. with rapid improvement in cough and no further syncopal episodes. The steroid was tapered over the next 2 weeks and at the 1-month follow-up visit, clinical evaluation and laryngoscopy were normal.
DISCUSSION
The mechanism of cough-related loss of consciousness has been debated since the first description in 1876. An early suggestion that it was a form of epilepsy was excluded in our case as direct electroencephalography during a spontaneous jerking attack demonstrated slowing of cerebral waves without critical activity. Another hypothesis was a rapid increase in liquor pressure with secondary circulatory arrest or cerebral concussion, but cerebral MRI excluded organic causes of altered liquor circulation. Continuous invasive pedidial blood pressure monitoring, avoiding jerk-related artefacts, showed a rapid reduction in blood pressure accompanied loss of consciousness, and demonstrated that the mechanism of syncope was related to a reduction in cerebral perfusion due to systemic hypotension[1]. Possible mechanisms of hypotension could be Valsalva related with a reduction in cardiac output, or rapid fluctuations in intrathoracic pressure during cough attacks that lead to a vaso-vagal reflex secondary to stimulation of aortic baroceptors[2].
In our case, the absence of syncope during Valsalva manoeuvre and the reproducible prevention of cough-induced syncope attacks by atropine suggested the second mechanism.
The question was why a patient without any past such history had suddenly developed vaso-vagal syncope. We demonstrated left vagal dysfunction with laryngoscopic evidence of left vocal cord hypomobility and subsequent laryngeal electromyography showed signs of neurogenic damage in the vagal-innervated muscles. The fact that the patient had dysphagia, dysphonia and dry cough, all possible symptoms of vagal neuropathy, further supported this hypothesis. We systematically excluded structural causes of vagal dysfunction by MRI and CT study of the cranial, cervical and thoracic nerves. Several cases of post-viral persistent vagal neuropathy are reported in the literature. We did not obtain biopsy specimens, but the rapid and sustained response to steroid therapy strongly suggests the presence of acute neuritis; negative follow-up laryngoscopy also confirmed an acute and transient event. To our knowledge this is the first report of acute vagal neuritis that manifested as malignant cough syncope with nerve dysfunction, possibly explaining both the cause of the cough and the mechanism of the secondary syncope.
This case of vaso-vagal reflex may have shown a pure vasodepressive response, as demonstrated by the absence of pacemaker intervention during syncope, because of the isolated left vagal dysfunction; vagal cardiac innervation is asymmetric, with only the right nerve going to the sino-atrial and atrio-ventricular nodes and inducing a cardio-inhibitory response. The most likely neural pathway to explain our case involves as afferent branch the Cyon nerve that carries signals from aortic arch baroceptors to the left vagal nerve. This hypothesis is further supported by the absence of carotid sinus hypersensitivity to neck massage, because in this second reflex the afferent branch is part the of glossopharyngeal nerve. Inflamed afferent nerve fibres may respond to a rise in intrathoracic pressure induced by intermittent cough with an increased baroceptor signal, leading to a severe vagal vasodepressive response.
While it was known that cough may be a cause of syncope and that vagal neuropathy may be a cause of persistent cough after respiratory viral infection, what it new in this case is the demonstration of acute left vagal dysfunction as the cause of pure vasodepressive vaso-vagal syncopes. Moreover, for the first time to our knowledge, acute vagal neuritis was strongly suggested by the rapid and sustained response to steroid therapy. The patient was discharged on oral prednisone 37.5 mg once daily with tapering over the following 2 weeks. Laryngoscopy showed normal cord mobility 1 month later and clinical follow-up was uneventful 2 months later.
In conclusion, cough syncope is an unusual form of situational syncope that may occur in both the standing and supine positions. Its rapid and unpredictable occurrence can have traumatic consequences. In the clinical evaluation of these patients, the first step is to connect loss of consciousness to cough and then to determine the aetiology of cough in order to establish specific treatment, exploring first the more common causes, such as gastroesophageal reflux, obstructive pulmonary disease and ACE inhibitors[3], and then uncommon causes, such as cerebral pathology or post-viral vagal neuropathy. If suspected, this entity can be initially investigated by fibrolaryngoscopy and confirmed by laryngeal electromyograph [4,5]. A trial of steroid therapy can be initiated.