ABSTRACT
Spinal cord haematoma, or haematomyelia, is a rare condition caused by several unusual disease processes. Traumatic events, such as spinal cord injury and surgery or procedures involving the spinal cord, are the most important causes of spinal cord haematoma. Rarely, it is associated with anticoagulation therapy. Irrespective of cause, spinal cord haematoma is considered a neurosurgical emergency and must be treated promptly in order to prevent neurological sequelae. The authors describe the case of a 69-year-old patient taking warfarin in the therapeutic range for a mechanic mitral valve, who developed chest pain with cervical and dorsal radiation, and experienced sudden paraparesis of the limbs. A CT of the spine confirmed haematomyelia. A high index of suspicion, prompt recognition and immediate intervention are essential to prevent major morbidity and mortality from intraspinal haemorrhage.
LEARNING POINTS
- This article reports an unusual presentation of spontaneous spinal haematoma, imposing the careful elaboration of differential diagnoses, which is very important in internal medicine.
- The description of this low-incidence case allows the scientific community to assist in approaching patients with similar symptoms.
- The lack of studies about the etiology and treatment of spontaneous spinal haematoma underlines the need for further studies and research in the area in order to increase the scientific evidence on the approach of these patients.
KEYWORDS
Haematomyelia, warfarin, laminectomy, epidural haematoma, spinal haematoma, spinal cord compression, myelopathy, spinal cord disease, neurologic deficit
INTRODUCTION
Spinal cord spontaneous haematoma (SCSH) is a rare condition which can cause rapid and irreversible neurological impairment. The incidence is reported to be one per 100,000 population per year and represents 0.3–0.9% of all spinal cord lesions[1–7]. The main causes implicated in SCSH are traumatic injuries or complications of spinal medical procedures such as lumbar puncture or epidural anaesthesia. In a few cases, other aetiologies may be involved, such as vascular malformations of the spinal cord, clotting disorders, inflammatory and infectious myelitis, spinal tumours and syringomyelia. However, in 40–50% of cases no aetiological cause can be found and the condition is considered spontaneous[2,8].
Patients with intramedullary spinal cord haematomas may present with severe spinal pain and significant neurological findings consistent with the level of spinal cord involvement. Sudden spinal pain at the level of the lesion is the first manifestation of SCSH (85%) and presents in some cases with metameric radiation[9]. In 92% of patients, neurological impairment appears simultaneously or sometimes after the pain has started, manifesting as pure sensory syndrome, pure motor hemiparesis or sensorimotor stroke. In some series, neurological impairment started 3.5 hours after the pain had begun[9]. MRI with and without gadolinium is still the investigation of choice for early diagnosis. Successful outcomes depend on early diagnosis, aggressive, emergent surgical treatment and drainage of the haematoma.
CASE DESCRIPTION
The authors describe the case of a 69-year-old patient who was admitted to the emergency department with a 6-hour history of epigastric pain without radiation and associated with acute cervical pain with lumbar radiation described as severe. The patient had previously been fitted with a mitral prosthetic valve and was being anticoagulated with warfarin. He denied nausea, vomiting, dyspnoea or other complaints. There was no recent history of trauma. During physical examination, the patient was found to be mildly anxious due to the pain. He was hypertensive with a blood pressure of 160/70 mmHg, heart rate of 70 bpm and a peripheral oxygen saturation of 98% without supplementation, and was afebrile. No relevant differential blood pressure between the arms and legs was observed. Cardiac, pulmonary, abdominal and vascular peripheral examinations were normal. There was no neurological impairment. The pain remained intense despite analgesia, including with morphine. The electrocardiogram and cardiac enzymes were normal. The echocardiogram was negative for valvular prosthetic dysfunction and for abnormalities of myocardial contraction. Blood analysis revealed a leucocyte count of 12,200/µl (normal: 4,000–10,000/µl) with 88.5% neutrophils, a haemoglobin of 15.9 g/dl (normal: 13.6–18.0 g/dl) and a platelet count of 189,000/µl (normal: 1,400,000–440,000/µl). Prothrombin time was 41.0 seconds, INR was 3.2 and D-dimers were 225.0 ng/ml (normal: 0–500 ng/ml). Creatinine was 0.77 mg/dl (normal: <0.9 mg/dl) and C-reactive protein was negative. A normal aortic angio-scan ruled out aortic disease.
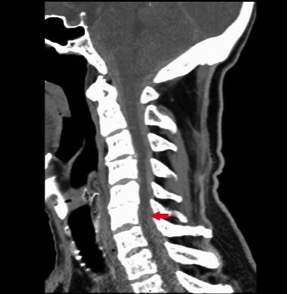
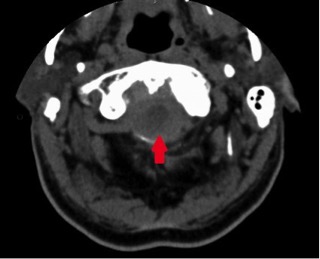
Four hours after admission, the patient experienced severe posterior thoracic pain without radiation and began to develop mild motor and sensory deficits in his lower extremities. Symptom severity rapidly progressed, and within 30 min the patient had paraparesis of the limbs (grade 2 in both legs), with bilateral sensory loss to the D1 dermatome level, with normal and symmetrical osteotendinous reflexes. The patient underwent an emergency medullar contrast CT scan which revealed a C5–D2 intracanalicular medullar haematoma (Figs. 1 and 2). At this point, the case was discussed with the neurosurgical team who decided on an emergency transfer to the reference centre for a decompression laminectomy. However, despite the prompt neurosurgical approach, the patient maintained paraparesis of the limbs after the procedure. After 2 months of physical rehabilitation with intensive physiotherapy, the patient achieved only minimal recovery, maintaining a high level of dependence in activities of daily living.
Figure 1. CT medullary scan showing a posterior medullar haematoma at the C5–T2 level (red arrow)
Figure 2. CT Medullar Scan showing posterior medullar hematoma at C5 level.
DISCUSSION
Spinal epidural haematomas were first described by Jackson in 1869[10] and first treated surgically by Bain in 1897[11]. The exact pathophysiology of SCSH is not clearly understood. Some authors maintain that the spontaneous haemorrhage is the result of increased intra-abdominal and intrathoracic pressure which is directly transmitted to the epidural venous plexus leading to rupture[12]. However, this suggestion is controversial because the absence of a valvular venous system at this level results in a low-pressure system. On the other hand, some authors believe that these ruptures have an arterial origin and are due rupture of the radicular arterioles which follow the course of the nerve roots, within the epidural space[13]. However in most case reports, no arterial rupture was seen when the haematoma was surgically removed[1,14]. SCSH usually affects patients between 40 and 80 years of age and has a male predominance (1.5 males for every female)[3,4]. Surgery and trauma are known risk factors for epidural haematomas, with other risk factors being less well-defined. Many authors suggest there is an association between SCSH and arteriovenous malformations, underlying coagulopathy, infectious and inflammatory medullar diseases and hypertension, while some reports indicate that 17–30% of SCSH is related to anticoagulant drug use. Other researchers have mentioned minor trauma, pregnancy, haemophilia and leukaemia as precipitating factors. In 40–60% of cases, there were no identifiable risk factors for haemorrhage[15–17]. In addition, a meta-analysis has suggested that individuals with hypertension do not have an increased risk of SCSH[17]. The cervicothoracic or thoracolumbar regions are the most frequent sites for SCSH with a posterior predominance. This is partly explained by the position of the anterior epidural veins which are anatomically supported by the posterior longitudinal ligament, associated with the fact that the posterior epidural plexus is larger than the ventral[15, 16]. There may also be an area of minor resistance that is more susceptible to rupture with small changes in intravenous pressure[15]. The neurological deficits are related to both compression and a secondary inflammatory reaction[14].
SCSHs have a wide spectrum of presentation ranging from radiculopathy to acute quadriplegia. Most patients present with severe back or neck pain, often with a radicular component[17]. The pain is usually followed by progressive motor or sensory deficits. Approximately 37% of patients present with complete sensorimotor deficits[8].
MRI is currently the imaging modality of choice for SCSH diagnosis, with CT of the spinal cord an option if MRI is not possible[3,4,18]. MRI of the spinal cord performed within 24 hours of symptom onset typically shows the haematoma as isointense on T1-weighted images and hyperintense on T2-weighted images[15,17,19]. After 24 hours, the haematoma often appears hyperintense on both T1- and T2-weighted images
Prompt treatment is one of the best predictors of a good outcome, and is optimal when delivered within 12–36 hours of symptom onset[2,20–23]. The neurological status of the patient prior to operative intervention is the most important prognostic indicator of long-term outcome[16]. The duration of symptoms, the degree of medullar compression[24] and the metamere impaired (worst in cervical and thoracic areas) are also prognostic factors[25]. Prompt neurosurgical treatment is best performed within the first 12–24 hours of motor impairment, although when motor deficits are minor, it can be delayed for 48 hours[5,8,26]. Some authors suggest that a surgical approach offers no benefits when motor impairment has existed for over 36 hours. The treatment of choice for SCSH typically is a hemilaminectomy or a laminectomy followed by irrigation and debridement[19,27,28]. In the last few years, some case reports describing conservative treatment (control of symptoms and corticoid therapy) have been published[3,8,20]. In some cases, serial MRIs have shown the blood has been reabsorbed, in one case within 4 months[29,30]. Candidate patients for conservative measures are those without neurological symptoms or those who recovered spontaneously within the first few hours of symptom onset. In such patients, meticulous assessment is necessary in order to identify patients at risk of a poor prognosis.
CONCLUSION
The rarity of this pathology increases the difficulty of examining underlying risk factors and further study is warranted. Physicians should be aware of the subtle signs of SCSH in the setting of minimal or no clear antecedent trauma and should initiate appropriate imaging and treatment. The authors hope with this case report to raise awareness of SCSH, especially in patients receiving anticoagulant therapy who complain of sudden and unexplained neck or back pain with acute myelopathy.