ABSTRACT
Heyde’s syndrome describes an association between aortic stenosis and a predisposition to bleeding from intestinal angiodysplasia resulting from acquired von Willebrand disease.
We present the case of an 82-year-old woman with recurrent intestinal bleeding, severe anaemia and secondary myocardial infarction. Investigation identified ileal angiectasia as the source of haemorrhage. As echocardiography revealed severe aortic stenosis the patient underwent surgical valve replacement. At her 3-month follow-up, the patient reported no new bleeding episodes and her functional status had improved.
This case highlights Heyde’s syndrome, an entity probably underdiagnosed despite the high prevalence of aortic stenosis and intestinal angiodysplasia in elderly patients.
LEARNING POINTS
- In a patient with bleeding intestinal angiectasia, Heyde’s syndrome should be considered in the differential diagnosis.
- Although supportive therapy is crucial for clinical stabilization, aortic valve replacement is the therapeutic gold standard.
- Appropriate management of these patients requires a multidisciplinary approach.
KEYWORDS
Aortic stenosis, intestinal angiodysplasia, Heyde’s syndrome, acquired von Willebrand disease
INTRODUCTION
The association between aortic stenosis and gastrointestinal haemorrhage was first described by Edward Heyde in 1958[1]. Further investigation confirmed a higher predisposition to bleeding from intestinal angiodysplasia in patients with severe aortic stenosis. This association became known as Heyde’s syndrome[2].
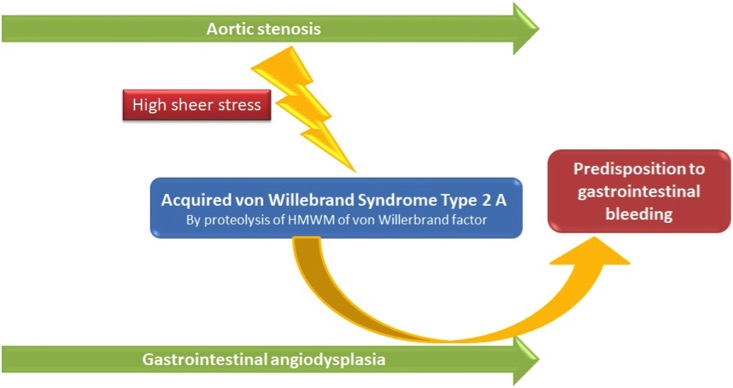
Heyde’s syndrome is believed to result from acquired von Willebrand disease (type 2A)[3]. Increased shear stress caused by aortic stenosis is thought to increase proteolysis and reduce the number of high molecular weight von Willebrand factor multimers, thus reducing platelet adhesion and generating a haemostatic defect (Fig. 1). The primary pathophysiological process connecting the two entities is a predisposition to bleeding from pre-existing gastrointestinal angiodysplasia. Aortic stenosis and intestinal angiodysplasia are frequent conditions in elderly patients, but the prevalence of their association is unclear and many cases may remain undiagnosed[4].
CASE DESCRIPTION
The authors present the case of an 82-year-old woman with a previous medical history of moderate aortic stenosis with congestive heart failure (New York Heart Association [NYHA] class III) and endometrial adenocarcinoma who had undergone surgical resection and radiotherapy the previous year.
The patient was first admitted to hospital due to melena with severe anaemia (Hb 5.4 g/dl, low iron and ferritin, and normal vitamin B12 and folic acid levels). She also complained of chest pain, with lateral wall repolarization abnormalities on electrocardiogram and elevated markers of myocardial necrosis consistent with a type II myocardial infarction, secondary to the severe anaemia.
Upper endoscopy and a thoraco-abdomino-pelvic computed tomography (CT) scan were normal. Colonoscopy revealed a bleeding sessile polyp 20 cm from the anal margin. The polyp was excised and local haemostatic measures were applied.
The patient was then discharged home asymptomatic and without evidence of further bleeding, with stable haemoglobin levels (8.3 g/dl) and normalization of cardiac biomarkers. However, she was readmitted 10 days later, again with melena and precordial pain, with severe anaemia (Hb 5.2 g/dl), electrocardiographic signs of ischaemia and elevation of cardiac markers (high sensitivity troponin I 7,324 pg/ml; reference <15.6 pg/ml).
During the patient’s first days on the medical ward, persistent and significant melena occurred, with haematological stability depending on vigorous transfusion therapy.
Melena suggested a proximal source of bleeding in the gastrointestinal tract, so upper gastrointestinal endoscopy was repeated, but results were normal. CT enterography revealed hyperdense fluid (probably blood) in the colon, but no inflammatory or tumoral lesions.
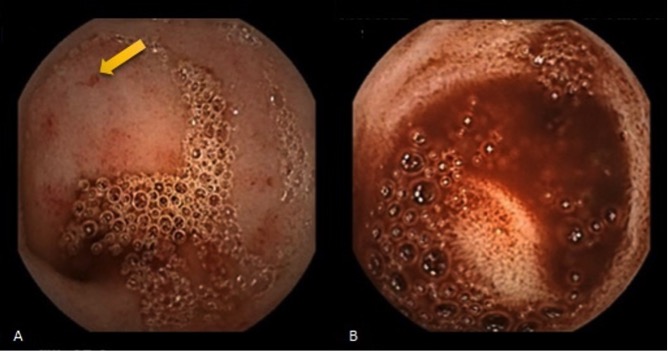
Capsule endoscopy identified angiectasia in the ileum (with blood in the surrounding lumen), presumably the source of the gastrointestinal bleeding (Fig. 2). An ileocolonoscopy was performed in an attempt to control the haemorrhage, but active bleeding lesions were not identified. At that time, clinical and haematological stability had been obtained.
Figure 1. Pathophysiological mechanism of Heyde’s syndrome. HMWM: high molecular weight multimers
Figure 2. (A) Blood and angiectasia (arrow) in the terminal ileum;
(B) blood in the caecum
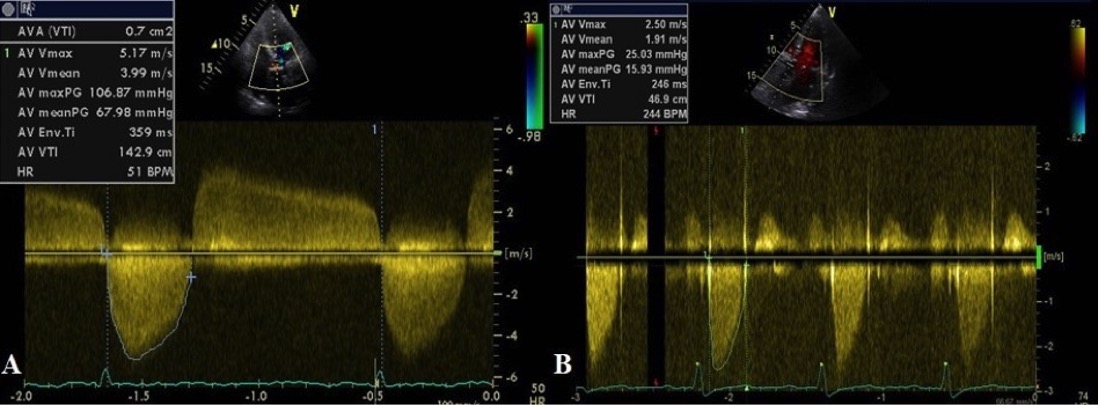
Following resolution of the bleeding, the patient’s cardiac condition was also controlled, and cardiac markers and electrocardiogram returned to normal. Coronary angiography was normal. Transthoracic echocardiography revealed worsened aortic stenosis, which was classified as severe (Fig. 3), and mild left ventricular dilation and hypertrophy.
Figure 3. Transthoracic echocardiogram
(A) before surgery, revealing severe aortic stenosis with a mean transaortic pressure gradient of 68 mmHg and aortic valve area of 0.7 cm2, and
(B) after valvular replacement, with normalization of the aortic gradient
Initial treatment was mostly supportive, with the priority being to ensure an adequate haemoglobin level through blood transfusions and replenishing iron stores. Invasive therapies for haemorrhagic control were not necessary. These approaches aim to control haemorrhage and its consequences, but the treatment gold standard is aortic valve replacement (the preferred method being surgical correction with a biological prosthesis).
The patient was discharged home but kept under close surveillance, and 1 month later underwent surgical aortic valve replacement with a biological prosthesis. A subsequent transthoracic echocardiogram showed adequate correction of the valvular defect (Fig. 3).
At her 3-month follow-up, the patient reported no new bleeding episodes and her functional status had improved significantly (having decreased from NYHA class III to class I). Haemoglobin levels had also stabilized (Hb 9–10 g/dl), with normal iron stores.
DISCUSSION
Investigation leading to the diagnosis in our patient was challenging, especially because of the need for multiple examinations before the identification of ileal angiectasia, presumed to be the bleeding starting point. A confounding factor was the finding of a bleeding colonic polyp, initially assumed to be the culprit, until the recurrence of bleeding after polypectomy and local haemostatic measures.
Although our patient had known aortic stenosis, Heyde’s syndrome was only considered after a transthoracic echocardiogram revealed a deteriorating condition, and following a thorough gastrointestinal investigation. This supports the previously mentioned idea that this syndrome is probably frequently overlooked and underdiagnosed.
In an elderly patient with recurrent gastrointestinal bleeding or anaemia of unclear origin, it is mandatory to first rule out structural lesions of the gastrointestinal tract. If the diagnosis remains uncertain, further studies considering the possibility of this syndrome may be sufficient, such as simple non-invasive transthoracic echocardiography to search for aortic stenosis.
Valvular correction reverses acquired von Willebrand disease, resolving gastrointestinal bleeding in up to 95% of cases[5]. The documented success in our patient, with complete recovery after valvular replacement, supports this diagnosis and emphasizes the value of high clinical suspicion.
Laboratory evidence of acquired von Willebrand disease would have been valuable to strengthen our hypothesis, but the required tests were unavailable.
A multidisciplinary approach is important in such cases, with the internal medicine, gastroenterology, cardiology and cardiac surgery departments making valuable contributions to the outcome in our patient.