ABSTRACT
Oral anticoagulant therapy is recommended for the prevention and treatment of venous thromboembolism and to prevent stroke in non-valvular atrial fibrillation. Until a few years ago, vitamin K antagonists were the only drugs available, but direct oral anticoagulants have recently been introduced into clinical practice for the same clinical indications. Unlike the situation with VKAs, fixed-dose administration was proposed for DOACs, without the necessity for routine laboratory monitoring. However, in clinical practice a high inter-variability in DOAC plasma levels, independently of the type of drug and patient characteristics, was observed and the importance of measuring DOAC anticoagulant activity to support treatment decisions has therefore been recognized. We describe two clinical cases in order to highlight the role and importance of dabigatran-specific measurements to guide patient management in case of major complications.
LEARNING POINTS
- Direct oral anticoagulants (DOACs) have been used in clinical practice at fixed doses without laboratory monitoring.
- However, the importance of measuring DOAC anticoagulant activity to support treatment decisions, particularly in emergency conditions, has been recognized.
- he clinical value of DOAC measurement is highlighted in the two described cases where the anticoagulation level was taken into consideration when deciding on treatment.
KEYWORDS
DOACs, dabigatran, anticoagulant, plasma measurement, idarucizumab
INTRODUCTION
Oral anticoagulant therapy is recommended for the prevention and treatment of venous thromboembolism and to prevent stroke in non-valvular atrial fibrillation (NVAF)[1]. Until few years ago, vitamin K antagonists (VKAs) were the only drugs available, but recently direct oral anticoagulants (DOACs) were introduced into clinical practice for the same clinical indications. DOACs include two classes of molecules with a similar pharmacological profile but with different pharmacokinetic and pharmacodynamic characteristics: an anti-IIa selective inhibitor (dabigatran) and anti-Xa selective inhibitors (rivaroxaban, apixaban and edoxaban). Unlike the situation with VKAs, fixed-dose administration was proposed for DOACs, based on the results of phase III clinical trials performed on selected populations, without the necessity for routine laboratory monitoring. However, in clinical practice a high inter-variability in DOAC plasma levels, independently of the type of drug and patient characteristics (gender, age, renal and liver function, and body weight) was observed[2]. Therefore, the importance of measuring DOAC anticoagulant activity to support treatment decisions, particularly in emergency conditions, has been recognized[3]. Dabigatran is a specific, reversible inhibitor of both free and clot-bound thrombin and its antidote (idarucizumab, Praxbind®) is available and recommended for patients who develop life-threatening or uncontrolled bleeding, or require an urgent surgical/invasive procedure. In these special clinical situations, the measurement of dabigatran plasma levels can guide[4-5] antidote use in the setting of acute bleeding, thus avoiding expensive and useless treatment.
We describe two clinical cases in order to highlight the role and importance of dabigatran-specific measurements to guide patient management in case of major complications.
CASE 1
A 58-year-old Caucasian woman, treated with dabigatran 150 mg twice daily for NVAF (CHA2S2-VASc score 4, HAS-BLED score 1) was admitted to the emergency department because of the sudden onset of left upper limb hyposthenia. Her medical history included heart failure, arterial hypertension, type 2 diabetes and obesity (body mass index >30).
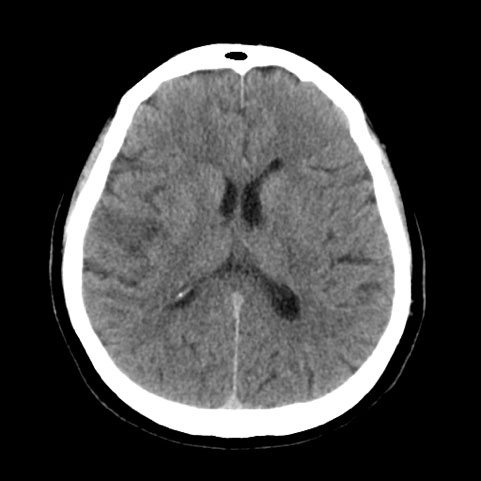
The neurological examination showed central left facial nerve paralysis, dysarthria mild dysphonia, distal motor deficit, apraxia, and tactile extinction in the left upper limb. At admittance, her blood pressure was 175/90 mmHg, National Institutes of Health Stroke Scale (NIHSS) was 6 and Glasgow Coma Score (GCS) was 15. A brain CT scan was negative for both bleeding and acute ischaemic lesions. Dabigatran measurements, performed on plasma samples using specific calibrated diluted thrombin time (dTT)[3], within 1 hour of admittance and 2 hours after administration of the last dose, were below the limit of quantification (dTT <30 ng /ml), showing the persistent absence of anticoagulant activity. After multidisciplinary evaluation by a neurologist and haemostasis/thrombosis expert, intravenous rt-PA thrombolysis was performed, within 4 hours of the onset of symptoms. A new CT scan 5 hours after the thrombolytic procedure showed right subcortical-cortical hypodensity of the frontal operculum, suggesting a stroke in the subacute phase (Fig. 1), without bleeding complications.
Figure 1. A CT brain scan, performed after the thrombolytic procedure, shows subcortical-cortical hypodensity of the right frontal operculum. Minimal residual contrast medium is seen in the posterior horn of the right lateral ventricle
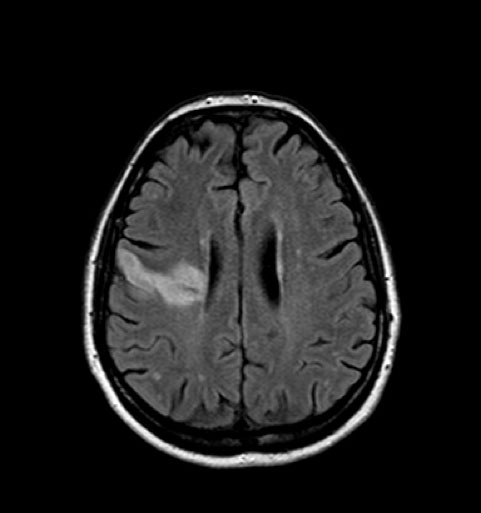
Figure 2. A brain NMR performed on day 5 after the thrombolytic procedure
On day 2, due to the high cardiovascular risk in an obese patient and the absence of bleeding, prophylactic treatment with enoxaparin plus warfarin was started. The patient was switched to VKA therapy because of the documented absence of dabigatran anticoagulant activity at admission (confirmed on two subsequent plasma samples), despite regular drug administration. In addition, due to the lack of evidence for DOAC treatment in obese patients, we rejected treatment with another DOAC.
The patient was discharged on warfarin in the therapeutic range (INR 2.0–3.0), in good clinical condition with minimal residual neurological deficits (mild facial nerve deficit and mild deficit in left-hand grip strength, NIHSS: 1).
CASE 2
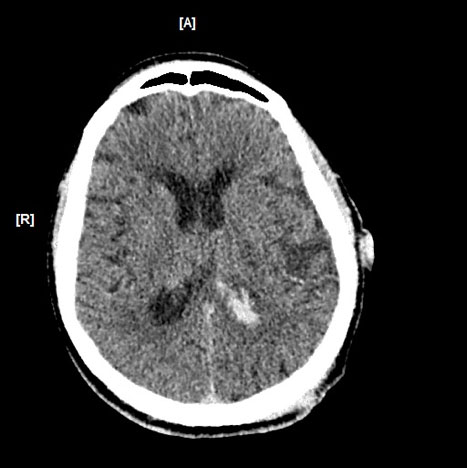
A 72-year-old man was admitted to the emergency department because of polytrauma due to a car crash which included severe brain injury and fracture of the left arm. The patient had NVAF (CHA2DS2-VASc score 3; HAS BLED score 2) and was on dabigatran 110 mg twice daily because of his medical history of a kidney stone complicated by several infections, right nephrectomy, myocardial infarction with coronary revascularization, arterial hypertension, chronic kidney disease and diabetes mellitus type 2. On admission, his blood pressure was 160/80 mmHg and his heart rate was 72 bpm, arrhythmic with normal saturation. His Glasgow Coma Score (GSC) was 15. Physical examination showed a large right parietal-temporal lacero-contusive injury. A brain CT scan revealed an interhemispheric haemorrhage and focal periventricular and frontal cortico-subcortical petechiae with minor intraventricular haemorrhage, without skull fractures (Fig. 3).
A total body CT scan excluded abdominal organ injury. An X-ray of the left arm showed non-linear radius and ulnar fractures.
Figure 3. A brain CT scan shows haemorrhage in the posterior horn of the left lateral ventricle
Dabigatran plasma levels were measured at admission as the patient’s neurological status suddenly worsened. The dTT was 188.6 ng/ml and idarucizumab was administered intravenously at the recommended dose of 5 g (2×2.5 g/50 ml)[4]. The dabigatran level was significantly lower than the limit of quantification (dTT: 2.28 ng/ml) 10 min after antidote administration, confirming complete reversal of the anticoagulant action. In light of the absolute contraindication to anticoagulant therapy in the first days after cerebral bleeding, graduated elastic compression stockings (18 mmHg) were immediately applied.
On day 5, as a new CT brain scan showed a reduction in subarachnoid and intraventricular haemorrhages, a prophylactic dose of low molecular weight heparin (LMWH) was started (nadroparin 3800 IU once daily for a patient weighing 70 kg). On day 12, the patient underwent orthopaedic surgery for the reduction and osteosynthesis of the displaced fracture of the left arm.
At discharge on day 21, the patient was conscious, oriented, without neurological deficits, and had been prescribed LMWH at a prophylactic dose.
As a brain CT scan, repeated 18 days after discharge, revealed complete resolution of the cerebral bleeds, the patient restarted anticoagulant treatment with dabigatran.
DISCUSSION
The two described cases show that measurement of dabigatran levels was crucial for guiding clinical management of the patients in light of the types of complications, possible requirement for fast reversal of anticoagulant activity, and oriented therapeutic approaches.
As shown in Case 1, specific dabigatran measurement allowed the administration of safe and timely thrombolytic therapy which was otherwise contraindicated by drug pharmacokinetics and the time since the last dose. In Case 2, after the presence of anticoagulant activity was confirmed, the specific reversal agent was correctly administered.
DOACs are now widely prescribed in clinical practice and, consequently, an increase in bleeding and thromboembolic complications is expected, which clinicians will have to manage correctly. However, the management of these conditions has not been properly standardized, clinicians lack experience[6] and only some case reports and case series have been reported in the literature[7].
In the setting of ischaemic stroke, patients treated with DOACs are frequently excluded from thrombolytic therapy because of the unpredictable risk of cerebral haemorrhage. According to the recent ASA/AHA guidelines[8], in the absence of appropriate laboratory tests, thrombolysis should not be administered unless DOACs were given to the patient >48 h previously. According to the ESO guidelines[9], in adult patients with acute ischaemic stroke (AIS) related to dabigatran and the suspicion or evidence of relevant drug concentrations, thrombolytic therapy cannot currently be recommended, denying a highly effective treatment although the presence of dabigatran is assumed only on the basis of time of last dose intake and renal function.
A recent study of 241 patients treated with rivaroxaban and experiencing either AIS or cerebral bleeding shows that half of the patients with AIS under rivaroxaban had a drug level low enough for thrombolysis to be considered, while a third of the ICH patients had a low rivaroxaban level and therefore did not require a specific reversal agent[10].
These recent publications confirm the usefulness of DOAC measurement, while our cases emphasize the added clinical value of anticoagulant activity evaluation. Specific DOACs measurements are now easily implementable in all hospitals and their availability should be advocated, even more so in emergency conditions.