Keywords
TRAPS syndrome, anakinra, allergic reaction, canakinumab
Abstract
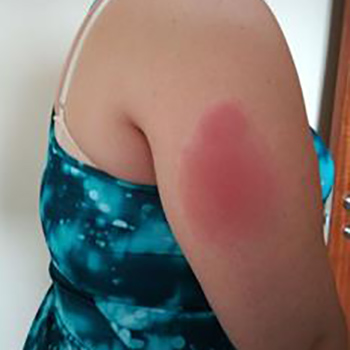
Tumor necrosis factor (TNF) receptor-associated periodic syndrome (TRAPS) is a rare hereditary systemic autoinflammatory disease (SAID). Treatment is based on corticosteroids, but often requires the addition of a biologic drug (anti-TNF agent, IL-1 receptor antagonist, etc) to achieve symptom control. The addition of the second drug is not clearly defined and must take into account the characteristics and preferences of the patient. We describe a patient with TRAPS and an allergic reaction to anakinra which was difficult to manage clinically while alternative treatment was being identified.
References
Views: 1121
HTML downloads: 927
PDF downloads: 506
Published:
2020-07-29
Issue:
2020: Vol 7 No 10
(view)










