Keywords
lymphoproliferative disorders, multicentric Castleman disease, siltuximab, IL-6, COVID-19
Abstract
Background: Castleman disease (CD) is a rare lymphoproliferative disorder with various subtypes, including the HHV-8-negative/idiopathic multicentric CD (iMCD). The diagnosis of iMCD remains challenging due to its non-specific presentation, in the form of generalised lymphadenopathies and inflammation. Two clinical presentations have been recently defined: a severe form iMCD-TAFRO and a milder form of iMCD not otherwise specified (iMCD-NOS). identification of interleukin-6 (IL-6) as a major culprit of inflammatory symptoms led to the development of anti-IL-6 therapies, with siltuximab being the approved first-line treatment.
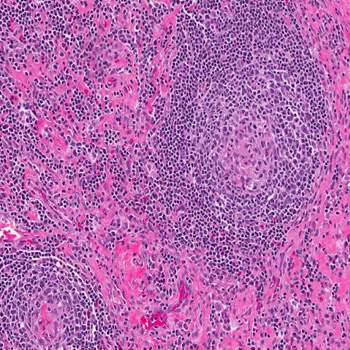
Case description: A 16-year-old male presented with recurrent fever, night sweats and several other non-specific symptoms. After extensive evaluations, an excisional lymph node biopsy confirmed the iMCD-NOS diagnosis. The patient received high-dose steroid therapy followed by siltuximab for four years. This treatment was well tolerated with only mild neutropenia not leading to dose adjustment. On siltuximab, the patient developed two mild COVID-19 episodes. His response to siltuximab remained effective throughout four years.
Discussion: The absence of biomarker or causal agent identification poses a diagnostic challenge requiring lymph node histopathology for a definitive diagnosis of iMCD. Anti-IL 6 (siltuximab) is the recommended frontline therapy, suppressing inflammation and halting disease progression. Intravenous administration every 3 to 6 weeks can impact patient quality of life, prompting further research for alternative treatments. High-dose steroids, rituximab, cyclosporine, tacrolimus, lenalidomide or combined chemotherapy such as rituximab-bortezomib-dexamethasone are among the considered options according to disease severity.
Conclusion: Overall, long-term siltuximab effectively controlled iMCD symptoms and was well tolerated by this young adult, who endured two mild COVID-19 episodes.
References
Views: 440
HTML downloads: 39
PDF downloads: 297
Published:
2023-11-17
Issue:
2023: Vol 10 No 12
(view)










