Keywords
Cabotegravir/Rilpivirine, Immune Reconstitution Inflammatory Syndrome, IRIS, AIDS, HIV
Abstract
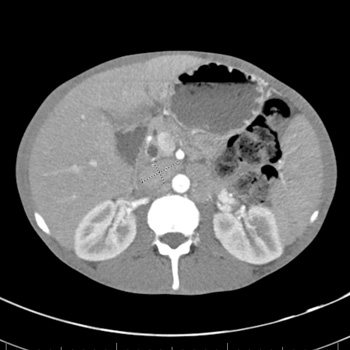
Long-acting (LA) cabotegravir/rilpivirine (CAB/RPV) is a complete regimen for the management of human immunodeficiency virus type 1 (HIV-1) infection to replace their oral antiretroviral therapy (ART) when they have been virologically suppressed. We present a case of successful achievement of undetectable HIV RNA viral load levels in an acquired immunodeficiency syndrome (AIDS) patient with long-standing virologic failure within two months of CAB/RPV LA initiation. This was later complicated by immune reconstitution inflammatory syndrome (IRIS) due to Mycobacterium avium-intracellulare (MAI) infection and hepatitis B virus (HBV) reactivation.
References
Views: 394
HTML downloads: 45
PDF downloads: 351
Published:
2023-07-13
Issue:
2023: Vol 10 No 8
(view)










